Textbook Question
The series of emission lines of the hydrogen atom for which nf = 3 is called the Paschen series. (b) Calculate the wavelengths of the first three lines in the Paschen series—those for which ni = 4, 5, and 6.
9
views

 Verified step by step guidance
Verified step by step guidance
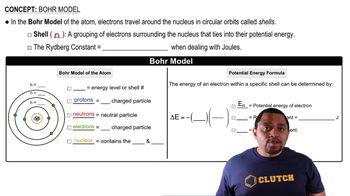
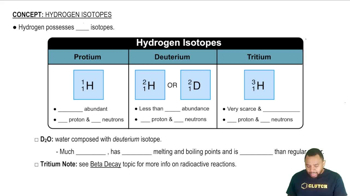

The series of emission lines of the hydrogen atom for which nf = 3 is called the Paschen series. (b) Calculate the wavelengths of the first three lines in the Paschen series—those for which ni = 4, 5, and 6.
Determine whether each of the following sets of quantum numbers for the hydrogen atom are valid. If a set is not valid, indicate which of the quantum numbers has a value that is not valid: (a) n = 3, l = 3, ml = 2, ms = +1/2 (b) n = 4, l = 3, ml = -3, ms = +1/2 (c) n = 3, l = 1, ml = 2, ms = +1/2 (d) n = 5, l = 0, ml = 0, ms = 0 (e) n = 2, l = 1, ml = 1, ms = -1/2