Determine whether each of the following sets of quantum numbers for the hydrogen atom are valid. If a set is not valid, indicate which of the quantum numbers has a value that is not valid: (a) n = 3, l = 3, ml = 2, ms = +1/2 (b) n = 4, l = 3, ml = -3, ms = +1/2 (c) n = 3, l = 1, ml = 2, ms = +1/2 (d) n = 5, l = 0, ml = 0, ms = 0 (e) n = 2, l = 1, ml = 1, ms = -1/2
Ch.6 - Electronic Structure of Atoms

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 6, Problem 95
An electron is accelerated through an electric potential to a kinetic energy of 1.6 * 10^-15 J. What is its characteristic wavelength? [Hint: Recall that the kinetic energy of a moving object is E = 1/2 mv^2, where m is the mass of the object and v is the speed of the object.]
 Verified step by step guidance
Verified step by step guidance1
Step 1: Identify the relevant equations. The de Broglie wavelength equation is \( \lambda = \frac{h}{p} \), where \( \lambda \) is the wavelength, \( h \) is Planck's constant, and \( p \) is the momentum of the electron. The momentum \( p \) can be expressed as \( p = mv \), where \( m \) is the mass and \( v \) is the velocity of the electron.
Step 2: Relate kinetic energy to velocity. The kinetic energy \( E \) of the electron is given by \( E = \frac{1}{2}mv^2 \). Rearrange this equation to solve for \( v \): \( v = \sqrt{\frac{2E}{m}} \).
Step 3: Calculate the momentum \( p \) using the velocity. Substitute the expression for \( v \) from Step 2 into the momentum equation \( p = mv \) to get \( p = m \sqrt{\frac{2E}{m}} \). Simplify this to \( p = \sqrt{2mE} \).
Step 4: Substitute the expression for momentum \( p \) into the de Broglie wavelength equation. This gives \( \lambda = \frac{h}{\sqrt{2mE}} \).
Step 5: Substitute known values into the equation. Use Planck's constant \( h = 6.626 \times 10^{-34} \text{ J s} \), the mass of an electron \( m = 9.109 \times 10^{-31} \text{ kg} \), and the given kinetic energy \( E = 1.6 \times 10^{-15} \text{ J} \) to calculate \( \lambda \).
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Kinetic Energy
Kinetic energy is the energy possessed by an object due to its motion, calculated using the formula E = 1/2 mv^2, where m is the mass and v is the velocity of the object. In this context, the kinetic energy of the electron is given as 1.6 * 10^-15 J, which will be used to determine its speed and subsequently its wavelength.
Recommended video:
Guided course
Kinetic & Potential Energy
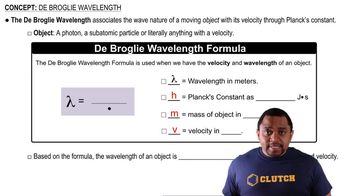
De Broglie Wavelength
The De Broglie wavelength is a concept in quantum mechanics that relates the wavelength of a particle to its momentum, expressed as λ = h/p, where h is Planck's constant and p is the momentum. For an electron, this wavelength can be calculated after determining its velocity from the kinetic energy, illustrating the wave-particle duality of matter.
Recommended video:
Guided course
De Broglie Wavelength Formula
Planck's Constant
Planck's constant (h) is a fundamental constant in quantum mechanics, approximately equal to 6.626 x 10^-34 Js. It plays a crucial role in the relationship between energy and frequency of photons, as well as in calculating the De Broglie wavelength of particles, linking classical and quantum physics.
Recommended video:
Guided course

Photons and Planck's Constant
Related Practice
Textbook Question
Textbook Question
Bohr's model can be used for hydrogen-like ions—ions that have only one electron, such as He+ and Li2+. (a) Why is the Bohr model applicable to He+ ions but not to neutral He atoms?
Textbook Question
As discussed in the A Closer Look box on 'Measurement and the Uncertainty Principle,' the essence of the uncertainty principle is that we can't make a measurement without disturbing the system that we are measuring. (a) Why can't we measure the position of a subatomic particle without disturbing it?
1
views
