A supersaturated solution of sucrose (C12H22O11) is made by dissolving sucrose in hot water and slowly letting the solution cool to room temperature. After a long time, the excess sucrose crystallizes out of the solution. Indicate whether each of the following statements is true or false: (b) After the excess sucrose has crystallized out, the system is now unstable and is not in equilibrium.
Ch.13 - Properties of Solutions

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 13, Problem 94a
The presence of the radioactive gas radon (Rn) in well water presents a possible health hazard in parts of the United States. (a) Assuming that the solubility of radon in water with 1 atm pressure of the gas over the water at 30 °C is 7.27⨉10-3 M, what is the Henry's law constant for radon in water at this temperature?
 Verified step by step guidance
Verified step by step guidance1
Identify the formula for Henry's Law, which is given by: \( C = k_H \cdot P \), where \( C \) is the concentration of the gas in the solution, \( k_H \) is the Henry's law constant, and \( P \) is the partial pressure of the gas.
Recognize that the problem provides the solubility of radon in water as \( 7.27 \times 10^{-3} \) M, which represents \( C \), and the pressure of radon over the water as 1 atm, which represents \( P \).
Substitute the given values into the Henry's Law equation: \( 7.27 \times 10^{-3} = k_H \cdot 1 \).
Solve for \( k_H \) by dividing both sides of the equation by the pressure (1 atm): \( k_H = \frac{7.27 \times 10^{-3}}{1} \).
Conclude that the Henry's law constant \( k_H \) for radon in water at 30 °C is equal to the solubility value, since the pressure is 1 atm.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Henry's Law
Henry's Law states that the amount of gas that dissolves in a liquid at a given temperature is directly proportional to the partial pressure of that gas above the liquid. This relationship can be expressed mathematically as C = kH * P, where C is the concentration of the gas in the liquid, kH is the Henry's law constant, and P is the partial pressure of the gas.
Recommended video:
Guided course
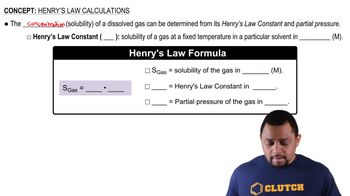
Henry's Law Calculations
Solubility
Solubility refers to the maximum amount of a substance that can dissolve in a solvent at a specific temperature and pressure. In the context of gases, solubility is influenced by factors such as temperature and pressure, with higher pressures generally increasing the solubility of gases in liquids.
Recommended video:
Guided course

Solubility Rules
Units of Henry's Law Constant
The Henry's law constant (kH) can be expressed in various units, depending on the context. Commonly, it is given in mol/(L·atm) or M/atm, indicating the concentration of the gas in moles per liter per unit of pressure in atmospheres. Understanding the units is crucial for correctly applying Henry's Law to calculate gas solubility.
Recommended video:
Guided course

Henry's Law Calculations
Related Practice
Textbook Question
Textbook Question
Most fish need at least 4 ppm dissolved O2 in water for survival. (a) What is this concentration in mol/L?
Textbook Question
Most fish need at least 4 ppm dissolved O2 in water for survival. (b) What partial pressure of O2 above water is needed to obtain 4 ppm O2 in water at 10 °C? (The Henry's law constant for O2 at this temperature is 1.71⨉10-3 mol/L-atm.)
Textbook Question
The presence of the radioactive gas radon (Rn) in well water presents a possible health hazard in parts of the United States. (b) A sample consisting of various gases contains 3.5 × 10-6 mole fraction of radon. This gas at a total pressure of 32 atm is shaken with water at 30 °C. Calculate the molar concentration of radon in the water.
