The 'free-base' form of cocaine (C17H21NO4) and its protonated hydrochloride form (C17H21NO4) are shown below; the free-base form can be converted to the hydrochloride form with one equivalent of HCl. For clarity, not all the carbon and hydrogen atoms are shown; each vertex represents a carbon atom with the appropriate number of hydrogen atoms so that each carbon makes four bonds to other atoms (e) How many mL of a concentrated 18.0 M HCl aqueous solution would it take to convert 1.00 kilograms (a 'kilo') of the free-base form of cocaine into its hydrochloride form?

Most fish need at least 4 ppm dissolved O2 in water for survival. (a) What is this concentration in mol/L?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Dissolved Oxygen Concentration
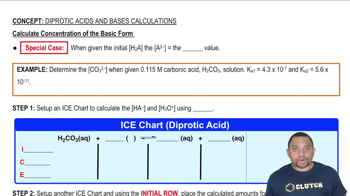
Molarity (mol/L)

Conversion between ppm and molarity

A supersaturated solution of sucrose (C12H22O11) is made by dissolving sucrose in hot water and slowly letting the solution cool to room temperature. After a long time, the excess sucrose crystallizes out of the solution. Indicate whether each of the following statements is true or false: (b) After the excess sucrose has crystallized out, the system is now unstable and is not in equilibrium.
Most fish need at least 4 ppm dissolved O2 in water for survival. (b) What partial pressure of O2 above water is needed to obtain 4 ppm O2 in water at 10 °C? (The Henry's law constant for O2 at this temperature is 1.71⨉10-3 mol/L-atm.)
The presence of the radioactive gas radon (Rn) in well water presents a possible health hazard in parts of the United States. (a) Assuming that the solubility of radon in water with 1 atm pressure of the gas over the water at 30 °C is 7.27⨉10-3 M, what is the Henry's law constant for radon in water at this temperature?
The presence of the radioactive gas radon (Rn) in well water presents a possible health hazard in parts of the United States. (b) A sample consisting of various gases contains 3.5 × 10-6 mole fraction of radon. This gas at a total pressure of 32 atm is shaken with water at 30 °C. Calculate the molar concentration of radon in the water.
