Textbook Question
(a) Describe what is meant by an electron-deficient molecule.

 Verified step by step guidance
Verified step by step guidance

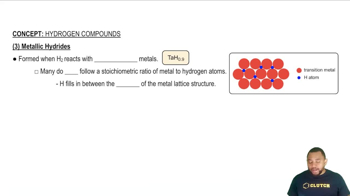

(a) Describe what is meant by an electron-deficient molecule.
(b) Describe what is meant by a three-center, two-electron bond.
Identify the group 4A element that best fits each of the following descriptions.
(b) Forms the strongest π bonds
Identify the group 4A element that best fits each of the following descriptions.
(c) Is the second most abundant element in the Earth's crust