We separate U-235 from U-238 by fluorinating a sample of uranium to form UF6 (which is a gas) and then taking advantage of the different rates of effusion and diffusion for compounds containing the two isotopes. Calculate the ratio of effusion rates for 238UF6 and 235UF6. The atomic mass of U-235 is 235.054 amu and that of U-238 is 238.051 amu.
Ch.6 - Gases

Chapter 6, Problem 96
A sample of CO2 effuses from a container in 55 seconds. How long will it take the same amount of gaseous Xe to effuse from the same container under identical conditions?
 Verified step by step guidance
Verified step by step guidance1
Identify the problem as one involving effusion, which can be solved using Graham's Law of Effusion.
Recall Graham's Law of Effusion: \( \frac{\text{Rate of effusion of gas 1}}{\text{Rate of effusion of gas 2}} = \sqrt{\frac{M_2}{M_1}} \), where \( M_1 \) and \( M_2 \) are the molar masses of the gases.
Set up the equation using the given information: \( \frac{\text{Rate of effusion of CO}_2}{\text{Rate of effusion of Xe}} = \sqrt{\frac{M_{\text{Xe}}}{M_{\text{CO}_2}}} \).
Recognize that the rate of effusion is inversely proportional to the time taken for effusion, so \( \frac{t_{\text{Xe}}}{t_{\text{CO}_2}} = \sqrt{\frac{M_{\text{Xe}}}{M_{\text{CO}_2}}} \).
Substitute the known values: \( t_{\text{CO}_2} = 55 \) seconds, \( M_{\text{CO}_2} = 44.01 \) g/mol, and \( M_{\text{Xe}} = 131.29 \) g/mol, and solve for \( t_{\text{Xe}} \).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Was this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Graham's Law of Effusion
Graham's Law states that the rate of effusion of a gas is inversely proportional to the square root of its molar mass. This means that lighter gases effuse faster than heavier gases. In this scenario, the effusion rates of CO2 and Xe can be compared using their molar masses to determine the time it will take for Xe to effuse.
Recommended video:
Guided course
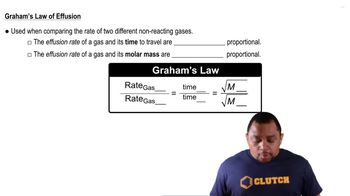
Graham's Law of Effusion
Molar Mass
Molar mass is the mass of one mole of a substance, typically expressed in grams per mole (g/mol). For this question, the molar masses of CO2 (approximately 44 g/mol) and Xe (approximately 131 g/mol) are crucial for applying Graham's Law. The difference in molar mass directly affects the effusion rates of the gases.
Recommended video:
Guided course

Molar Mass Concept
Effusion
Effusion is the process by which gas molecules escape from a container through a small opening into a vacuum or lower pressure area. The rate of effusion is influenced by factors such as temperature and the size of the gas molecules. Understanding effusion is essential for solving the problem, as it involves comparing the escape rates of CO2 and Xe.
Recommended video:
Guided course
Effusion Rate Example
Related Practice
Textbook Question
Textbook Question
Calculate the ratio of effusion rates for Ar and Kr.
Textbook Question
A sample of argon effuses from a container in 112 seconds. The same amount of an unknown noble gas requires 79.6 seconds. Identify the second gas.
Textbook Question
The graph shows the distribution of molecular velocities for two different molecules (A and B) at the same temperature. Which molecule has the higher molar mass? Which molecule has the higher rate of effusion?
2
views
Textbook Question
The graph shows the distribution of molecular velocities for the same molecule at two different temperatures (T1 and T2). Which temperature is greater? Explain.
Textbook Question
Which postulate of the kinetic molecular theory breaks down under conditions of high pressure? Explain.
