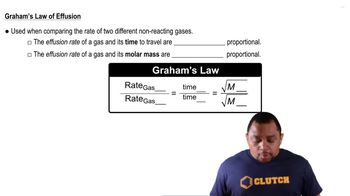
Calculate the ratio of effusion rates for Ar and Kr.

The graph shows the distribution of molecular velocities for two different molecules (A and B) at the same temperature. Which molecule has the higher molar mass? Which molecule has the higher rate of effusion?

 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
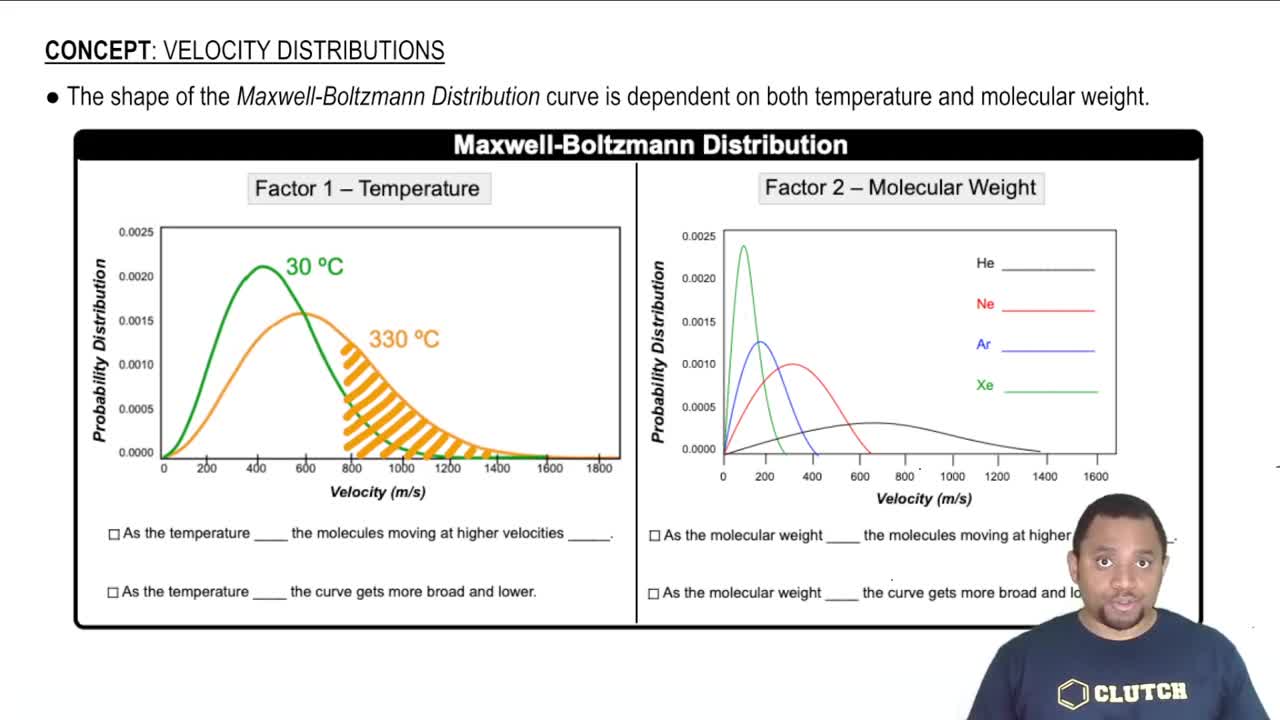
Molecular Velocity Distribution
Graham's Law of Effusion
Kinetic Molecular Theory

A sample of argon effuses from a container in 112 seconds. The same amount of an unknown noble gas requires 79.6 seconds. Identify the second gas.
A sample of CO2 effuses from a container in 55 seconds. How long will it take the same amount of gaseous Xe to effuse from the same container under identical conditions?
The graph shows the distribution of molecular velocities for the same molecule at two different temperatures (T1 and T2). Which temperature is greater? Explain.
Which postulate of the kinetic molecular theory breaks down under conditions of high pressure? Explain.
Use the van der Waals equation and the ideal gas equation to calculate the volume of 1.000 mol of neon at a pressure of 500.0 atm and a temperature of 355.0 K. Explain why the two values are different. (Hint: One way to solve the van der Waals equation for V is to use successive approximations. Use the ideal gas law to get a preliminary estimate for V.)
