Textbook Question
Two isomers of the planar compound 1,2-dichloroethylene are shown here.
(a) Which of the two isomers will have the stronger dipole– dipole forces?

 Verified step by step guidance
Verified step by step guidance
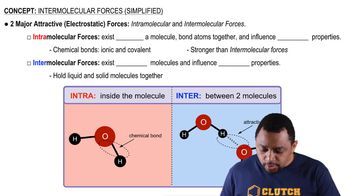
Two isomers of the planar compound 1,2-dichloroethylene are shown here.
(a) Which of the two isomers will have the stronger dipole– dipole forces?
The table below shows the normal boiling points of benzene and benzene derivatives.
(a) How many of these compounds exhibit dispersion interactions?
The table below shows the normal boiling points of benzene and benzene derivatives. (e) Why is the boiling point of phenol the highest of all?
(a) When you exercise vigorously, you sweat. How does this help your body cool?
(b) A flask of water is connected to a vacuum pump. A few moments after the pump is turned on, the water begins to boil. After a few minutes, the water begins to freeze. Explain why these processes occur.