
Water has a high heat-absorbing capacity because ________.
a. The sun's rays penetrate to the bottom of bodies of water, mainly heating the bottom surface.
b. The strong covalent bonds that hold individual water molecules together require large inputs of heat to break.
c. It has the ability to dissolve many heat-resistant solutes.
d. Initial energy inputs are first used to break hydrogen bonds between water molecules and only after these are broken, to raise the temperature; e. all of the above are true.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Heat Capacity

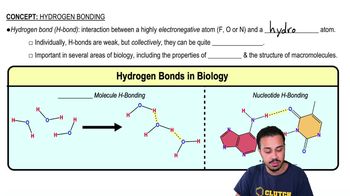
Hydrogen Bonds
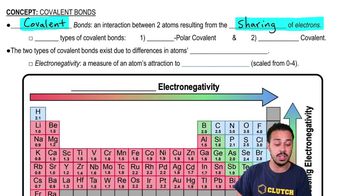
Covalent Bonds
Carbon dioxide functions as a greenhouse gas by ________.
a. Interfering with water's ability to absorb heat.
b. Increasing the random molecular motions of oxygen.
c. Allowing radiation from the sun to reach Earth and absorbing the re-radiated heat.
d. Splitting into carbon and oxygen and increasing the rate of cellular respiration.
The burning of fossil fuels ________.
a. Releases carbon dioxide to the atmosphere.
b. Primarily occurs as a result of human activity.
c. Is contributing to global warming.
d. Is possible thanks to photosynthesis that occurred millions of years ago.
e. All of the above are correct.
Stomata on a plant's surface ________.
a. Prevent oxygen from escaping.
b. Produce water as a result of photosynthesis.
c. Cannot be regulated by the plant.
d. Allow carbon dioxide uptake into leaves.
e. Are found in stacks called thylakoids.
Which of the following does not occur during the light reactions of photosynthesis?
a. Water is released.
b. Electrons from chlorophyll are moved to a higher-energy state by light.
c. ATP is produced.
d. NADPH is produced to carry electrons to the light-independent reactions.
e. Oxygen is produced when water is split.
