Textbook Question
List the four biological molecules commonly found in living organisms.
6
views

 Belk, Maier 6th Edition
Belk, Maier 6th Edition Ch. 2 - Science Fiction, Bad Science, and Pseudoscience
Ch. 2 - Science Fiction, Bad Science, and Pseudoscience Problem 3
Problem 3
 Verified step by step guidance
Verified step by step guidance

List the structural features in a prokaryotic cell.
Water ________.
a. Is a good solute.
b. Facilitates chemical reactions.
c. Serves as an enzyme.
d. Makes strong covalent bonds with other molecules.
e. Consists of two oxygen and one hydrogen atoms.
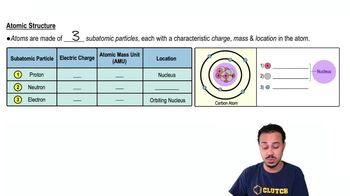
Electrons ________.
a. Are negatively charged.
b. Along with neutrons make up the nucleus.
c. Are attracted to the negatively charged nucleus.
d. Are not involved in ionic bonds.
e. All of the above are true.
Which of the following terms is least like the others?
a. Monosaccharide
b. Phospholipid
c. Fat
d. Steroid
e. Lipid