6. Chemical Equilibrium
Solubilty Product Constant
6. Chemical Equilibrium
Solubilty Product Constant
Showing 10 of 10 videos
Practice this topic
- Multiple Choice
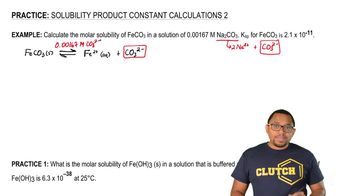
What is the molar solubility of Fe(OH) 3 (s) in a solution that is buffered at pH 3.50 at 25°C? The Ksp of Fe(OH)3 is 6.3 x 10–38 at 25°C.
- Multiple Choice
Arsenic trisulfide (As2S3) occurs naturally as the orange-yellow colored mineral orpiment. As2S3 is a highly insoluble substance with a Ksp value of 2.90×10−72. Calculate the solubility of As2S3 in g/100mL. (Molar mass of As2S3 = 246.04 g/mol)