- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

An unknown compound gives a weak molecular ion peak at m/z99 in the mass spectrum. Its NMR spectrum is shown here. The IR spectrum has shown a weak peak at 2255 cm-1, a strong peak at 1750 cm-1, and a strong peak at 1205 cm-1.
Determine its structure and propose a suitable fragmentation for the MS peak at m/z 68.

Propose the structure of an unknown compound using the NMR spectrum and the additional data provided here. The elemental analysis confirmed the molecular formula of the compound to be C8H7OBr. The IR spectrum shows two stretched at 1692 cm−1and 1605 cm−1. The mass spectrum shows a double molecular ion peak with equal intensities at m/z198 and 200.
The MS and NMR spectra along with some prominent IR peaks of an unknown compound are given below. Propose a structure consistent with the given data. Also, show the fragments that give the prominent peaks at m/z 105 and 77.
IR peaks: 1685 cm−1 and 1610 cm−1


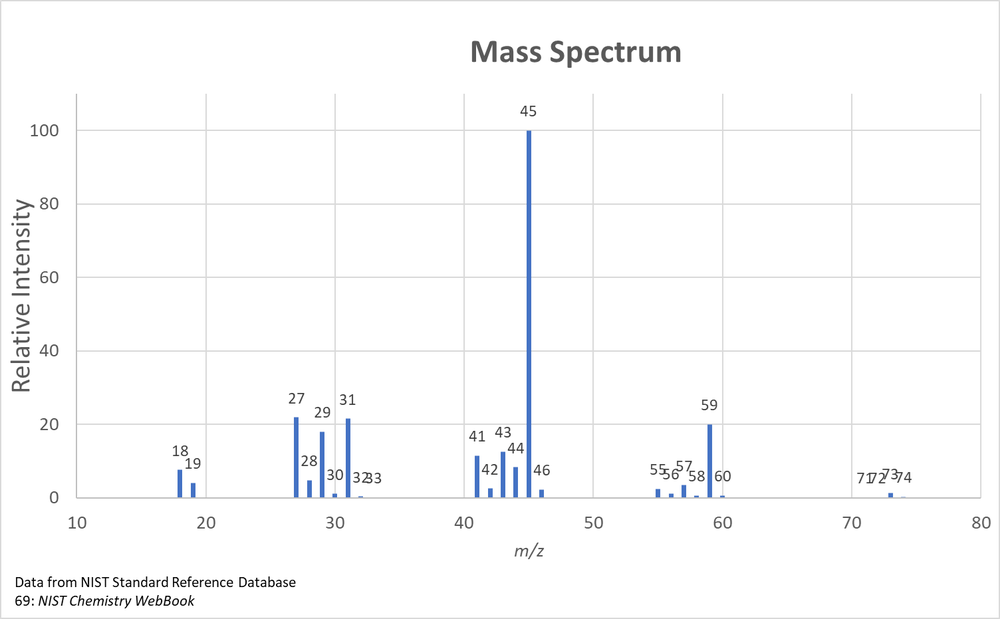
Shown below is the mass spectrum of butan-2-ol. Identify the m/z of the fragments generated from one dehydration and two α-cleavage fragmentations.
For the given compound, draw the structure of any one major fragment expected to appear in its mass spectrum.

In the following mass spectrum, identify the peaks that represent theα-cleavage of heptan-3-one.

The mass spectrum of unbranched primary alcohols often shows a peak at M–46. Determine the fragment/s lost that caused this peak.