Give the correct IUPAC name for each of the following compounds:
(b)

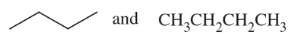
 Verified step by step guidance
Verified step by step guidance

Give the correct IUPAC name for each of the following compounds:
(b)
What is the difference between a conformational isomer of a compound and a structural isomer of the same compound?
Determine the relationship between each of the pairs of the following compounds. Are they structural isomers (different molecules), conformational isomers (the same molecule), or not related?
(b)
Determine the relationship between each of the pairs of the following compounds. Are they structural isomers (different molecules), conformational isomers (the same molecule), or not related?
(d)
Determine if each of the following cycloalkanes or alkenes can exist as cis–trans stereoisomers. For those that can, draw the two isomers. Label each of the isomers you drew as the cis stereoisomer or the trans stereoisomer.
(b) CH3CH2CH2CH2CH=CH2
Mark the chiral centers in the following molecules, if any, with an asterisk (*):
(a)