Textbook Question
Write the Lewis structure for each molecule. d. CH3SH (C and S central)


 Verified step by step guidance
Verified step by step guidance


Write the Lewis structure for each molecule. d. CH3SH (C and S central)
Write the Lewis structure for each molecule. a. CH2O b. C2Cl4 c. CH3NH2 d. CFCl3 (C central)
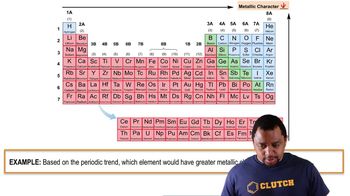
Determine if a bond between each pair of atoms would be pure covalent, polar covalent, or ionic. a. Ba and O
Draw the Lewis structure for ClF with an arrow representing the dipole moment.
Refer to Figure 10.10 to estimate the percent ionic character of the ClF bond.
Write the Lewis structure for each molecule or ion. b. OH-