Formic acid is responsible for the sting of ant bites. By mass, formic acid is 26.10% C, 4.38% H, and 69.52% O. The molar mass of formic acid is 46.02 g/mol. Determine the molecular formula of formic acid and draw its Lewis structure.
Ch.10 - Chemical Bonding I: The Lewis Model

Chapter 10, Problem 100
NaCl has a lattice energy of -787 kJ/mol. Consider a hypothetical salt XY. X3+ has the same radius of Na+ and Y3- has the same radius as Cl-. Estimate the lattice energy of XY.
 Verified step by step guidance
Verified step by step guidance1
insert step 1: Understand the concept of lattice energy, which is the energy required to separate one mole of an ionic solid into its gaseous ions. It is influenced by the charges of the ions and the distance between them.
insert step 2: Recognize that the lattice energy is proportional to the product of the charges of the ions and inversely proportional to the distance between them. This can be expressed as: \( U \propto \frac{q_1 \cdot q_2}{r} \), where \( q_1 \) and \( q_2 \) are the charges of the ions, and \( r \) is the distance between the ions.
insert step 3: Compare the charges of the ions in NaCl and XY. In NaCl, Na+ has a charge of +1 and Cl- has a charge of -1. In XY, X3+ has a charge of +3 and Y3- has a charge of -3.
insert step 4: Calculate the ratio of the product of the charges for XY to that of NaCl. For NaCl, the product is \( (+1) \times (-1) = -1 \). For XY, the product is \( (+3) \times (-3) = -9 \). The ratio is \( \frac{-9}{-1} = 9 \).
insert step 5: Estimate the lattice energy of XY by multiplying the lattice energy of NaCl by the ratio of the charge products. Since the lattice energy of NaCl is -787 kJ/mol, the estimated lattice energy of XY would be \( -787 \times 9 \) kJ/mol.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
5mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Lattice Energy
Lattice energy is the amount of energy released when gaseous ions combine to form an ionic solid. It is a measure of the strength of the forces between the ions in an ionic compound. A more negative lattice energy indicates a more stable ionic compound, as it reflects stronger ionic interactions. The lattice energy can be estimated using the charges and sizes of the ions involved.
Recommended video:
Guided course

Lattice Energy
Ionic Radii
Ionic radii refer to the effective size of an ion in a crystal lattice. The size of an ion affects the distance between ions in the lattice, which in turn influences the lattice energy. For example, smaller ions can pack more closely together, leading to stronger electrostatic attractions and higher lattice energy. Understanding the ionic radii of the ions involved is crucial for estimating lattice energy.
Recommended video:
Guided course
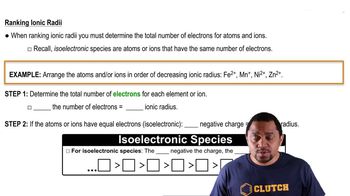
Ranking Ionic Radii
Coulomb's Law
Coulomb's Law describes the electrostatic interaction between charged particles. It states that the force between two charged ions is directly proportional to the product of their charges and inversely proportional to the square of the distance between them. This principle is fundamental in calculating lattice energy, as it helps to quantify the strength of the ionic bonds based on the charges and distances of the ions in the lattice.
Recommended video:
Guided course
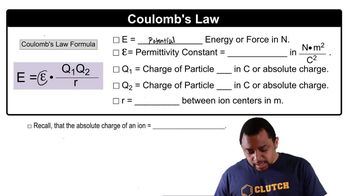
Coulomb's Law Concept 2
Related Practice
Textbook Question
Textbook Question
Diazomethane is a highly poisonous, explosive compound because it readily evolves N2. Diazomethane has the following composition by mass: 28.57% C; 4.80% H; and 66.64% N. The molar mass of diazomethane is 42.04 g/mol. Find the molecular formula of diazomethane, draw its Lewis structure, and assign formal charges to each atom. Why is diazomethane not very stable? Explain.
Textbook Question
The reaction of Fe2O3(s) with Al(s) to form Al2O3(s) and Fe(s) is called the thermite reaction and is highly exothermic. What role does lattice energy play in the exothermicity of the reaction?
Textbook Question
Draw the Lewis structure for each organic compound from its condensed structural formula. b. CH3OCH3
