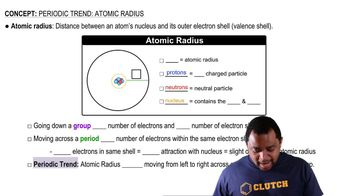
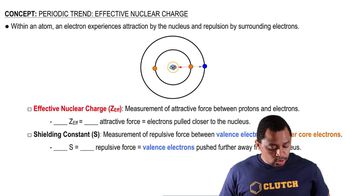
Atomic Radius
Atomic radius refers to the size of an atom, typically measured from the nucleus to the outermost electron shell. As you move across a period in the periodic table, the atomic radius generally decreases due to the increasing positive charge of the nucleus, which pulls the electrons closer, resulting in a smaller atomic size.

 Verified step by step guidance
Verified step by step guidance