Textbook Question
Determine the energy of 1 mol of photons for each kind of light. (Assume three significant figures.) a. infrared radiation (1500 nm) b. visible light (500 nm) c. ultraviolet radiation (150 nm)
1
views

 Verified step by step guidance
Verified step by step guidance

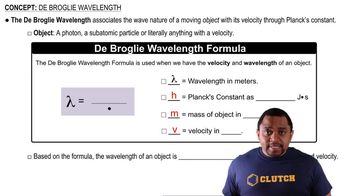

Determine the energy of 1 mol of photons for each kind of light. (Assume three significant figures.) a. infrared radiation (1500 nm) b. visible light (500 nm) c. ultraviolet radiation (150 nm)
How much energy is contained in 1 mol of each? a. X-ray photons with a wavelength of 0.135 nm b. γ-ray photons with a wavelength of 2.15×10–5 nm
Sketch the interference pattern that results from the diffraction of electrons passing through two closely spaced slits.
The smallest atoms can themselves exhibit quantum-mechanical behavior. Calculate the de Broglie wavelength (in pm) of a hydrogen atom traveling at 475 m/s.
A proton in a linear accelerator has a de Broglie wavelength of 132 pm. What is the speed of the proton?