An electron in the n = 7 level of the hydrogen atom relaxes to a lower-energy level, emitting light of 397 nm. What is the value of n for the level to which the electron relaxed?

The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. The change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. The minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kJ/mol. Calculate the longest wavelength visible to the human eye.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Energy and Wavelength Relationship

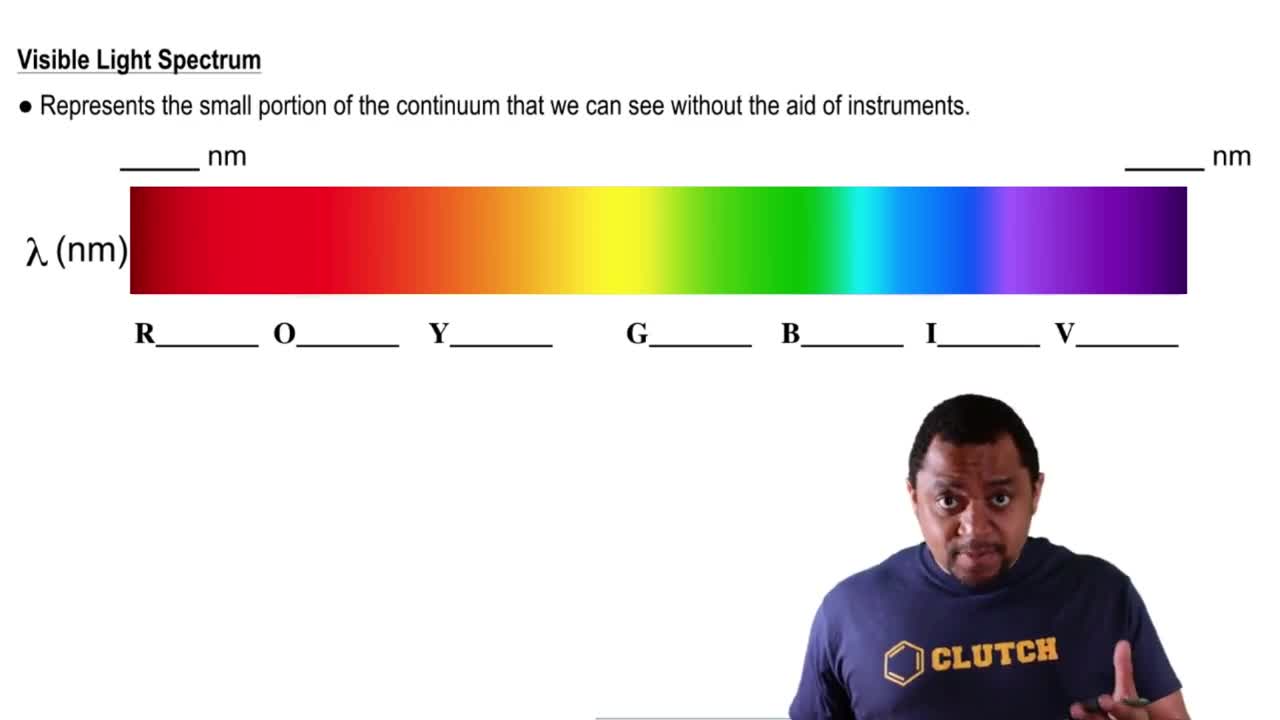
Visible Spectrum
Conformational Change in Molecules
An electron in a hydrogen atom relaxes to the n = 4 level, emitting light of 114 THz. What is the value of n for the level in which the electron originated?
Ultraviolet radiation and radiation of shorter wavelengths can damage biological molecules because these kinds of radiation carry enough energy to break bonds within the molecules. A typical carbon–carbon bond requires 348 kJ/mol to break. What is the longest wavelength of radiation with enough energy to break carbon–carbon bonds?
An argon ion laser puts out 5.0 W of continuous power at a wavelength of 532 nm. The diameter of the laser beam is 5.5 mm. If the laser is pointed toward a pinhole with a diameter of 1.2 mm, how many photons travel through the pinhole per second? Assume that the light intensity is equally distributed throughout the entire cross-sectional area of the beam. (1 W = 1 J/s)
