An atomic emission spectrum of hydrogen shows three wavelengths: 1875 nm, 1282 nm, and 1093 nm. Assign these wavelengths to transitions in the hydrogen atom.

In order for a thermonuclear fusion reaction of two deuterons (2^1H^+) to take place, the deuterons must collide and each must have a velocity of about 1 * 10^6 m/s. Find the wavelength of such a deuteron.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
De Broglie Wavelength
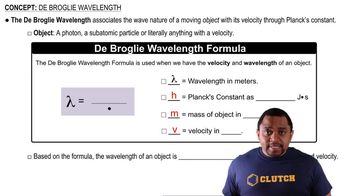
Momentum

Thermonuclear Fusion

An atomic emission spectrum of hydrogen shows three wavelengths: 121.5 nm, 102.6 nm, and 97.23 nm. Assign these wavelengths to transitions in the hydrogen atom.
The speed of sound in air is 344 m/s at room temperature. The lowest frequency of a large organ pipe is 30 s–1 and the highest frequency of a piccolo is 1.5×104 s–1. Find the difference in wavelength between these two sounds.
The distance from Earth to the sun is 1.5×108 km. Find the number of crests in a light wave of frequency 1.0×1014 s –1 traveling from the sun to Earth.
A 5.00-mL ampule of a 0.100-M solution of naphthalene in hexane is excited with a flash of light. The naphthalene emits 12.3 J of energy at an average wavelength of 349 nm. What percentage of the naphthalene molecules emitted a photon?
