Textbook Question
Convert between energy units. c. 4.99 × 103 kJ to kWh
8
views

 Verified step by step guidance
Verified step by step guidance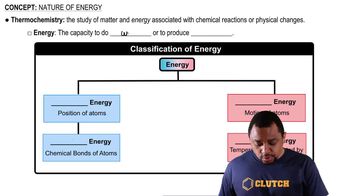


Convert between energy units. c. 4.99 × 103 kJ to kWh
Suppose that a person eats 2105 Calories per day. Convert this amount of energy into each unit. c. kWh
A particular frost-free refrigerator uses about 705 kWh of electrical energy per year. Express this amount of energy in each unit. d. Cal