Textbook Question
Calculate the molarity of each solution. c. 132.4 mg NaCl in 255.4 mL of solution

 Verified step by step guidance
Verified step by step guidance


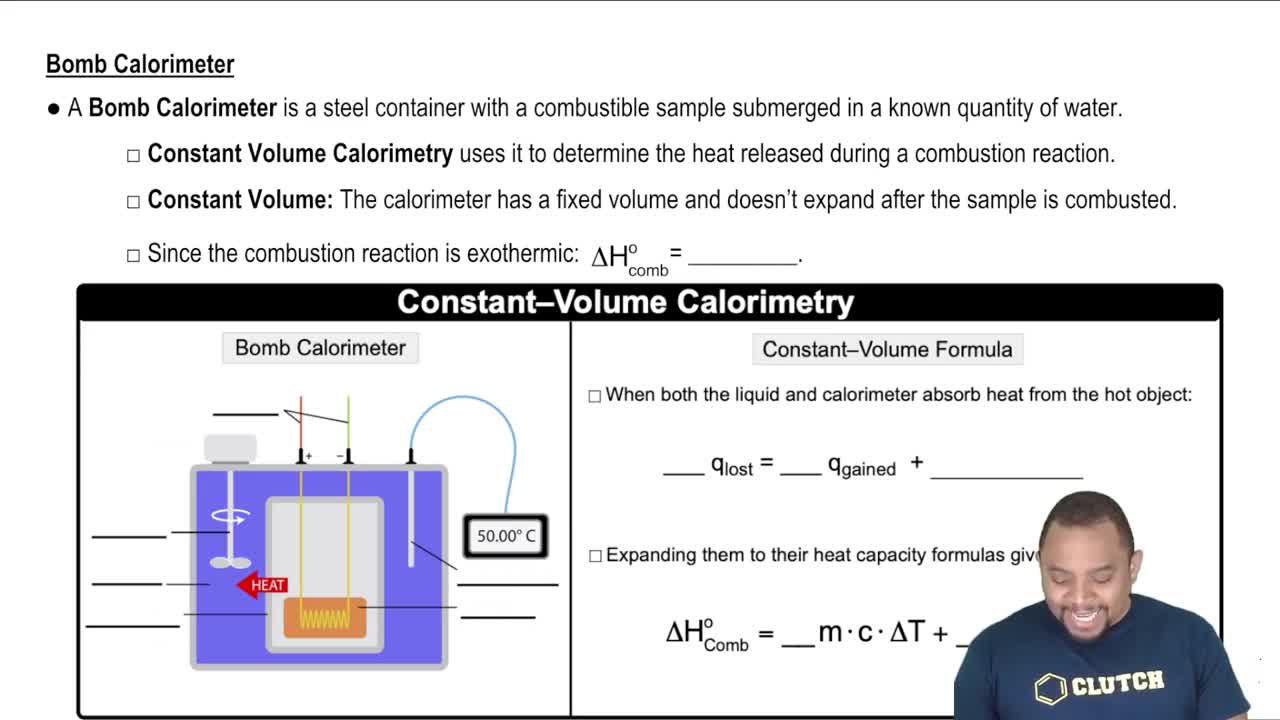
Calculate the molarity of each solution. c. 132.4 mg NaCl in 255.4 mL of solution
What is the molarity of NO3– in each solution? a. 0.150 M KNO3 b. 0.150 M Ca(NO3)2 c. 0.150 M Al(NO3)3
What is the molarity of NO3– in each solution? a. 0.225 M NaNO3 b. 0.225 M Mg(NO3)2 c. 0.225 M Al(NO3)3