Aerosol cans carry clear warnings against incineration because of the high pressures that can develop upon heating. Suppose that a can contains a residual amount of gas at a pressure of 755 mmHg and a temperature of 25 °C. What would the pressure be if the can were heated to 1155 °C?
Ch.6 - Gases

Chapter 6, Problem 60
Use the molar volume of a gas at STP to calculate the density (in g/L) of carbon dioxide gas at STP.
 Verified step by step guidance
Verified step by step guidance1
Identify the molar volume of a gas at STP, which is 22.4 L/mol.
Determine the molar mass of carbon dioxide (CO_2). Carbon has a molar mass of 12.01 g/mol and oxygen has a molar mass of 16.00 g/mol. Therefore, CO_2 has a molar mass of 12.01 + 2(16.00) g/mol.
Use the formula for density: \( \text{Density} = \frac{\text{Mass}}{\text{Volume}} \).
Substitute the molar mass of CO_2 for the mass and the molar volume (22.4 L) for the volume in the density formula.
Calculate the density of CO_2 in g/L by dividing the molar mass by the molar volume.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Molar Volume of a Gas
The molar volume of a gas at standard temperature and pressure (STP) is the volume occupied by one mole of an ideal gas, which is approximately 22.4 liters. This value is crucial for calculations involving gases, as it allows for the conversion between moles and volume, facilitating the determination of various properties such as density.
Recommended video:
Guided course
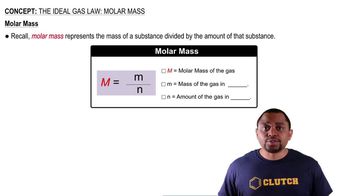
The Ideal Gas Law: Molar Mass
Density of a Gas
Density is defined as mass per unit volume, typically expressed in grams per liter (g/L) for gases. To calculate the density of a gas, one can use the formula: density = mass/volume. Understanding how to derive the mass of carbon dioxide from its molar mass and the volume it occupies at STP is essential for this calculation.
Recommended video:
Guided course

Gas Density Example
Standard Temperature and Pressure (STP)
Standard Temperature and Pressure (STP) refers to a set of conditions commonly used in gas calculations, defined as 0 degrees Celsius (273.15 K) and 1 atmosphere of pressure. At STP, the behavior of gases can be predicted using the ideal gas law, and it provides a reference point for comparing gas properties, including density and molar volume.
Recommended video:
Guided course
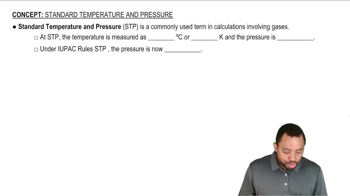
Standard Temperature and Pressure
Related Practice
Textbook Question
1
views
Textbook Question
A sample of nitrogen gas in a 1.75-L container exerts a pressure of 1.35 atm at 25 °C. What is the pressure if the volume of the container is maintained constant and the temperature is raised to 355 °C?
Textbook Question
Use the molar volume of a gas at STP to determine the volume (in L) occupied by 27.2 g of argon at STP.
Textbook Question
What is the density (in g/L) of hydrogen gas at 20.0 °C and a pressure of 1655 psi?
2
views
Textbook Question
A sample of N2O gas has a density of 2.85 g/L at 298 K. What is the pressure of the gas (in mmHg)?
Textbook Question
A 248-mL gas sample has a mass of 0.433 g at a pressure of 745 mmHg and a temperature of 28 °C. What is the molar mass of the gas?
