A gaseous hydrogen- and carbon-containing compound is decomposed and found to contain 82.66% carbon and 17.34% hydrogen by mass. The mass of 158 mL of the gas, measured at 556 mmHg and 25 °C, was 0.275 g. What is the molecular formula of the compound?
Ch.6 - Gases

Chapter 6, Problem 112
Consider the reaction: 2 Ag2O(s) → 4 Ag(s) + O2(g) If this reaction produces 15.8 g of Ag(s), what total volume of gas can be collected over water at a temperature of 25 °C and a total pressure of 752 mmHg?
 Verified step by step guidance
Verified step by step guidance1
Identify the balanced chemical equation: \(2 \text{Ag}_2\text{O}(s) \rightarrow 4 \text{Ag}(s) + \text{O}_2(g)\).
Calculate the moles of Ag produced using its molar mass: \(\text{moles of Ag} = \frac{15.8 \text{ g}}{\text{molar mass of Ag}}\).
Use stoichiometry to find the moles of \(\text{O}_2\) produced: \(\text{moles of O}_2 = \frac{1}{4} \times \text{moles of Ag}\).
Use the ideal gas law to find the volume of \(\text{O}_2\) at the given conditions: \(PV = nRT\), where \(P\) is the pressure of \(\text{O}_2\) (corrected for water vapor), \(V\) is the volume, \(n\) is the moles of \(\text{O}_2\), \(R\) is the ideal gas constant, and \(T\) is the temperature in Kelvin.
Adjust the total pressure to account for water vapor pressure at 25 °C, then solve for the volume of \(\text{O}_2\).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
7mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Stoichiometry
Stoichiometry is the calculation of reactants and products in chemical reactions based on the balanced equation. It allows us to determine the amount of substances consumed and produced in a reaction. In this case, knowing the amount of silver produced helps us find the moles of oxygen gas generated, which is essential for further calculations.
Recommended video:
Guided course

Stoichiometry Concept
Ideal Gas Law
The Ideal Gas Law relates the pressure, volume, temperature, and number of moles of a gas through the equation PV = nRT. This law is crucial for calculating the volume of gas produced in the reaction. By rearranging the equation, we can solve for volume when we know the number of moles of gas and the conditions of temperature and pressure.
Recommended video:
Guided course

Ideal Gas Law Formula
Dalton's Law of Partial Pressures
Dalton's Law states that the total pressure of a gas mixture is equal to the sum of the partial pressures of each individual gas. When collecting gas over water, the vapor pressure of water must be considered. This means that the pressure of the gas collected must be adjusted by subtracting the vapor pressure of water at the given temperature to find the pressure of the dry gas.
Recommended video:
Guided course
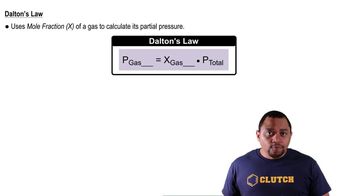
Dalton's Law and Partial Pressure
Related Practice
Textbook Question
Textbook Question
A gaseous hydrogen- and carbon-containing compound is decomposed and found to contain 85.63% C and 14.37% H by mass. The mass of 258 mL of the gas, measured at STP, was 0.646 g. What is the molecular formula of the compound?
Textbook Question
Consider the reaction:
2 SO2(g) + O2(g) → 2 SO3(g)
a. If 285.5 mL of SO2 reacts with 158.9 mL of O2 (both measured at 315 K and 50.0 mmHg), what is the limiting reactant and the theoretical yield of SO3?
Textbook Question
Ammonium carbonate decomposes upon heating according to the balanced equation: (NH4)2CO3(s) → 2 NH3(g) + CO2(g) + H2O(g) Calculate the total volume of gas produced at 22 °C and 1.02 atm by the complete decomposition of 11.83 g of ammonium carbonate.
