A sample of gas has a mass of 38.8 mg. Its volume is 224 mL at a temperature of 55 °C and a pressure of 886 torr. Find the molar mass of the gas.

A gas mixture with a total pressure of 745 mmHg contains each of the following gases at the indicated partial pressures: CO2, 112 mmHg; Ar, 225 mmHg; and O2, 114 mmHg. The mixture also contains helium gas. What is the partial pressure of the helium gas? What mass of helium gas is present in a 12.0-L sample of this mixture at 273 K?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
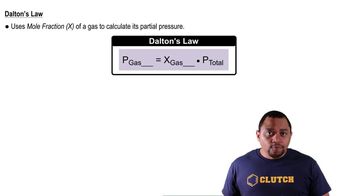
Dalton's Law of Partial Pressures
Ideal Gas Law

Molar Mass

A sample of gas has a mass of 0.555 g. Its volume is 117 mL at a temperature of 85 °C and a pressure of 753 mmHg. Find the molar mass of the gas.
A gas mixture contains each of the following gases at the indicated partial pressures: N2, 111 torr; O2, 213 torr; and He, 102 torr. What is the total pressure of the mixture? What mass of each gas is present in a 1.55-L sample of this mixture at 25.0°C?
A 1.20-g sample of dry ice is added to a 755 mL flask containing nitrogen gas at a temperature of 25.0 °C and a pressure of 725 mmHg. The dry ice sublimes (converts from solid to gas), and the mixture returns to 25.0 °C. What is the total pressure in the flask?
A 275-mL flask contains pure helium at a pressure of 752 torr. A second flask with a volume of 475 mL contains pure argon at a pressure of 722 torr. If we connect the two flasks through a stopcock and we open the stopcock, what is the partial pressure of argon?
A gas mixture contains 1.05 g N2 and 1.35 g O2 in a 1.35-L container at 15°C. Calculate the mole fraction and partial pressure of each component in the gas mixture.
