Binary compounds of alkali metals and hydrogen react with water to liberate H2(g). The H2 from the reaction of a sample of NaH with an excess of water fills a volume of 0.490 L above the water. The temperature of the gas is 35 °C and the total pressure is 758 mmHg. Determine the mass of H2 liberated and the mass of NaH that reacted.
Ch.6 - Gases

Chapter 6, Problem 136
A sample of N2O3(g) has a pressure of 0.017 atm. The temperature (in K) is doubled and the N2O3 undergoes complete decomposition to NO2(g) and NO(g). Find the total pressure of the mixture of gases assuming constant volume and no additional temperature change.
 Verified step by step guidance
Verified step by step guidance1
Identify the initial conditions: The initial pressure of N2O3 is 0.017 atm.
Write the balanced chemical equation for the decomposition: N2O3(g) → NO2(g) + NO(g).
Determine the mole ratio from the balanced equation: 1 mole of N2O3 produces 1 mole of NO2 and 1 mole of NO.
Apply the ideal gas law concept: Since the volume is constant and temperature is doubled, the pressure of each gas will also double.
Calculate the total pressure: Add the pressures of NO2 and NO, considering the initial pressure of N2O3 and the effect of temperature doubling.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Ideal Gas Law
The Ideal Gas Law relates the pressure, volume, temperature, and number of moles of a gas through the equation PV = nRT. This law is essential for understanding how changes in temperature and pressure affect gas behavior. In this scenario, the initial pressure of N2O3 and the effects of temperature doubling on gas pressure are analyzed using this principle.
Recommended video:
Guided course

Ideal Gas Law Formula
Stoichiometry of Gas Reactions
Stoichiometry involves the calculation of reactants and products in chemical reactions. For the decomposition of N2O3 into NO2 and NO, the stoichiometric coefficients indicate the molar relationships between the gases produced. Understanding these relationships is crucial for determining the total pressure of the gas mixture after the reaction.
Recommended video:
Guided course

Gas Stoichiometry Concepts
Dalton's Law of Partial Pressures
Dalton's Law states that the total pressure of a gas mixture is equal to the sum of the partial pressures of each individual gas. This concept is vital for calculating the total pressure after the decomposition of N2O3, as it allows for the addition of the partial pressures of NO2 and NO produced from the reaction, assuming no volume change.
Recommended video:
Guided course
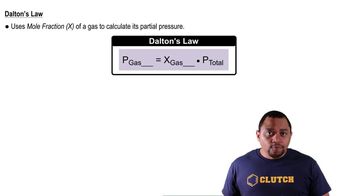
Dalton's Law and Partial Pressure
Related Practice
Textbook Question
Textbook Question
In a given diffusion apparatus, 15.0 mL of HBr gas diffuses in 1.0 min. In the same apparatus and under the same conditions, 20.3 mL of an unknown gas diffuses in 1.0 min. The unknown gas is a hydrocarbon. Find its molecular formula.
Textbook Question
A gas mixture composed of helium and argon has a density of 0.670 g/L at a 755 mmHg and 298 K. What is the composition of the mixture by volume?
Textbook Question
A gas mixture contains 75.2% nitrogen and 24.8% krypton by mass. What is the partial pressure of krypton in the mixture if the total pressure is 745 mmHg?
1
views
