Calculate the empirical formula for each natural flavor based on its elemental mass percent composition. a. methyl butyrate (component of apple taste and smell): C 58.80%, H 9.87%, O 31.33%
Ch.3 - Molecules and Compounds

Chapter 3, Problem 99
The elemental mass percent composition of ibuprofen (a nonsteroidal anti-inflammatory drug [NSAID]) is 75.69% C, 8.80% H, and 15.51% O. Determine the empirical formula of ibuprofen.
 Verified step by step guidance
Verified step by step guidance1
Convert the given mass percentages of each element into grams assuming a 100 g sample. This means you have 75.69 g of C, 8.80 g of H, and 15.51 g of O.
Calculate the number of moles of each element by dividing the mass of each element by its atomic mass: use 12.01 g/mol for C, 1.008 g/mol for H, and 16.00 g/mol for O. Express this as for each element.
Determine the mole ratio of the elements by dividing each element's mole value by the smallest number of moles calculated among the elements.
If the mole ratios are not whole numbers, multiply all ratios by the smallest factor that converts them into whole numbers (e.g., 2, 3, 4, etc.). This will give the subscripts for each element in the empirical formula.
Write the empirical formula by assigning the whole number mole ratios as subscripts to the respective elements C, H, and O.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Mass Percent Composition
Mass percent composition expresses the percentage by mass of each element in a compound. It is used to determine the relative amounts of elements present, which is essential for calculating the empirical formula by converting these percentages into moles.
Recommended video:
Guided course

Mass Percent Calculation
Mole Concept and Conversion
The mole concept allows conversion from mass to moles using atomic masses. By dividing the mass percent of each element by its atomic mass, you find the mole ratio of elements, which is crucial for determining the simplest whole-number ratio in the empirical formula.
Recommended video:
Guided course

Mole Concept
Empirical Formula Determination
The empirical formula represents the simplest whole-number ratio of atoms in a compound. After calculating moles of each element, divide all mole values by the smallest mole number to find the ratio, then round to the nearest whole number to write the empirical formula.
Recommended video:
Guided course
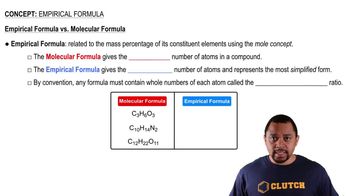
Empirical vs Molecular Formula
Related Practice
Textbook Question
2
views
Textbook Question
Calculate the empirical formula for each stimulant based on its elemental mass percent composition.
a. nicotine (found in tobacco leaves): C 74.03%, H 8.70%, N 17.27%
b. caffeine (found in coffee beans): C 49.48%, H 5.19%, N 28.85%, O 16.48%
3
views
Textbook Question
Calculate the empirical formula for each natural flavor based on its elemental mass percent composition. b. vanillin (responsible for the taste and smell of vanilla): C 63.15%, H 5.30%, O 31.55%
3
views
Textbook Question
The elemental mass percent composition of ascorbic acid (vitamin C) is 40.92% C, 4.58% H, and 54.50% O. Determine the empirical formula of ascorbic acid.
1
views
Textbook Question
A 45.2-mg sample of phosphorus reacts with selenium to form 131.6 mg of the selenide. Determine the empirical formula of phosphorus selenide.
