Textbook Question
Calculate the mass (in g) of each sample. a. 4.5×1025 O3 molecules
3
views

 Verified step by step guidance
Verified step by step guidance

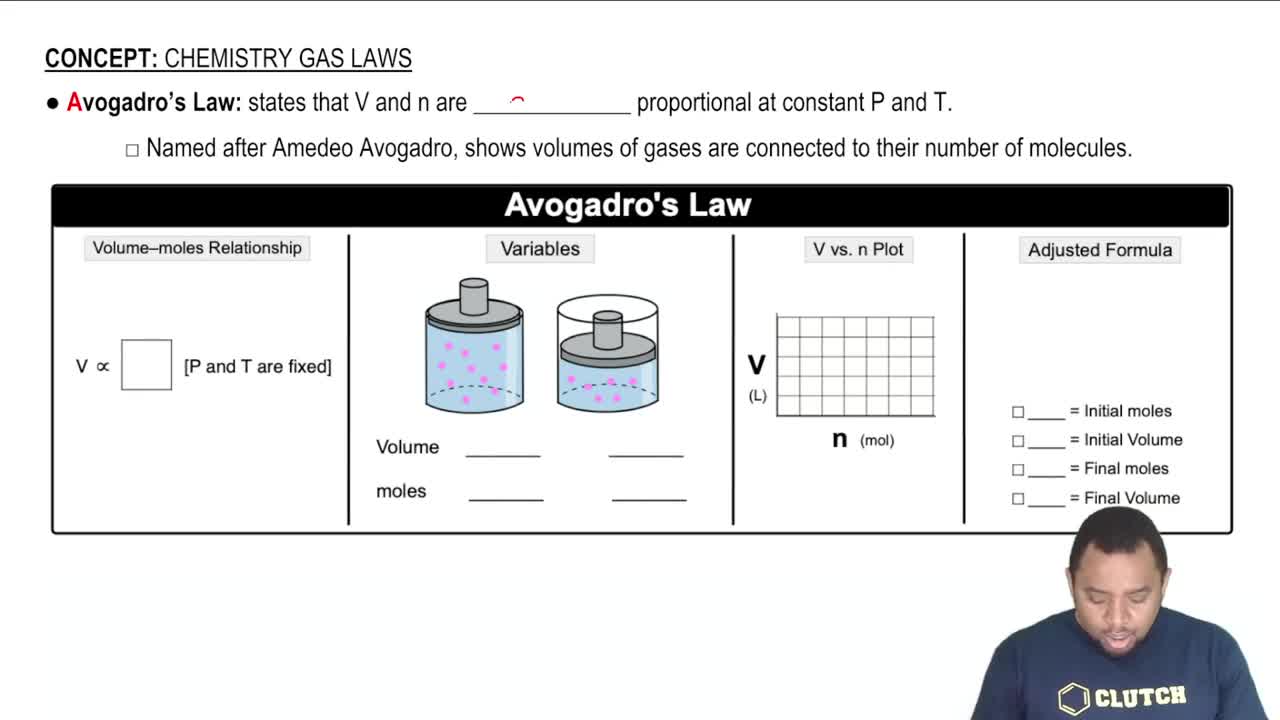

Calculate the mass (in g) of each sample. a. 4.5×1025 O3 molecules
Calculate the mass (in g) of each sample. b. 9.85×1019 CCl2F2 molecules c. 1 water molecule
A salt crystal has a mass of 0.27 mg. How many NaCl formula units does it contain?
Calculate the mass percent composition of carbon in each carbon-containing compound. a. CH4 b. C2H6
Calculate the mass percent composition of carbon in each carbon-containing compound. c. C2H2
Calculate the mass percent composition of carbon in each carbon-containing compound. d. C2H5Cl