Textbook Question
Based on the molecular formula, determine whether each compound is an alkane, alkene, or alkyne. (Assume that the hydrocarbons are noncyclical and there is no more than one multiple bond.)
a. C5H12 b. C2H2 c. C7H14 d. C11H22

 Verified step by step guidance
Verified step by step guidance
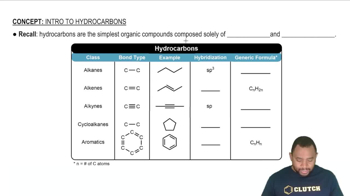

Based on the molecular formula, determine whether each compound is an alkane, alkene, or alkyne. (Assume that the hydrocarbons are noncyclical and there is no more than one multiple bond.)
a. C5H12 b. C2H2 c. C7H14 d. C11H22
Write structural formulas for each of the nine structural isomers of heptane.
Write structural formulas for any 6 of the 18 structural isomers of octane.