Rutherfordium-257 was synthesized by bombarding Cf-249 with C-12. Write the nuclear equation for this reaction.
Ch.21 - Radioactivity & Nuclear Chemistry

Chapter 21, Problem 68
A typical home uses approximately 1.0⨉103 kWh of energy per month. If the energy came from a nuclear reaction, what mass would have to be converted to energy per year to meet the energy needs of the home?
 Verified step by step guidance
Verified step by step guidance1
Identify the total energy consumption per year by multiplying the monthly energy usage by 12 months: \(1.0 \times 10^3 \text{kWh/month} \times 12 \text{ months/year}\).
Convert the energy from kilowatt-hours (kWh) to joules (J) using the conversion factor: \(1 \text{ kWh} = 3.6 \times 10^6 \text{ J}\).
Use Einstein's mass-energy equivalence formula \(E = mc^2\) to find the mass \(m\) that would be converted to energy, where \(E\) is the energy in joules and \(c\) is the speed of light \(3.0 \times 10^8 \text{ m/s}\).
Rearrange the formula to solve for mass \(m\): \(m = \frac{E}{c^2}\).
Substitute the calculated energy \(E\) and the speed of light \(c\) into the equation to find the mass \(m\) in kilograms.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Energy-Mass Equivalence
Energy-mass equivalence is a principle derived from Einstein's theory of relativity, expressed by the equation E=mc². This equation states that energy (E) and mass (m) are interchangeable; a small amount of mass can be converted into a large amount of energy due to the speed of light squared (c²) being a very large number. This concept is crucial for understanding how mass can be converted to meet energy needs.
Recommended video:
Guided course
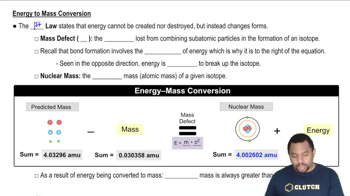
Energy to Mass Conversion
Nuclear Energy
Nuclear energy is the energy released during nuclear reactions, such as fission or fusion. In fission, heavy atomic nuclei split into smaller parts, releasing energy, while fusion involves light nuclei combining to form a heavier nucleus. Understanding nuclear energy is essential for calculating how much mass must be converted to meet energy demands, as it provides a context for the energy output from nuclear reactions.
Recommended video:
Guided course

Nuclear Binding Energy
Energy Consumption Calculations
Energy consumption calculations involve determining the total energy used over a specific period, often measured in kilowatt-hours (kWh). In this scenario, the monthly energy usage of 1.0⨉10³ kWh must be converted to an annual figure to assess the total energy requirement. This calculation is fundamental for quantifying the energy needs of a household and subsequently relating it to the mass of fuel required for energy production.
Recommended video:
Guided course

Gibbs Free Energy of Reactions
Related Practice
Textbook Question
Textbook Question
If 1.0 g of matter is converted to energy, how much energy is formed?
Textbook Question
Calculate the mass defect and nuclear binding energy per nucleon of each nuclide. a. Li-7 (atomic mass = 7.016003 amu)
Textbook Question
Calculate the quantity of energy produced per gram of U-235 (atomic mass = 235.043922 amu) for the neutron-induced fission of U-235 to form Xe-144 (atomic mass = 143.9385 amu) and Sr-90 (atomic mass = 89.907738 amu) (discussed in Problem 57).
Textbook Question
Calculate the quantity of energy produced per mole of U-235 (atomic mass = 235.043922 amu) for the neutron-induced fission of U-235 to produce Te-137 (atomic mass = 136.9253 amu) and Zr-97 (atomic mass = 96.910950 amu) (discussed in Problem 58).
