Textbook Question
The nuclide 6Li reacts with 2H to form two identical particles. Identify the particles.

 Verified step by step guidance
Verified step by step guidance
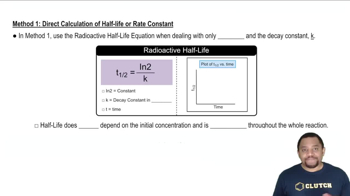
The nuclide 6Li reacts with 2H to form two identical particles. Identify the particles.
The half-life of 238U is 4.5⨉109 yr. A sample of rock of mass 1.6 g produces 29 dis/s. Assuming all the radioactivity is due to 238U, find the percent by mass of 238U in the rock.
The half-life of 232Th is 1.4⨉1010 yr. Find the number of disintegrations per hour emitted by 1.0 mol of 232Th.