A hydrogen-filled balloon is ignited and 3 g of hydrogen is reacted with 24 g of oxygen. How many grams of water vapor form? (Assume that water vapor is the only product.)
Ch.2 - Atoms & Elements

Chapter 2, Problem 33
Two samples of carbon tetrachloride are decomposed into their constituent elements. One sample produces 38.9 g of carbon and 448 g of chlorine, and the other sample produces 14.8 g of carbon and 134 g of chlorine. Are these results consistent with the law of definite proportions? Explain your answer.
 Verified step by step guidance
Verified step by step guidance1
Identify the law of definite proportions, which states that a chemical compound always contains the same proportion of elements by mass.
Calculate the mass ratio of chlorine to carbon for the first sample: divide the mass of chlorine (448 g) by the mass of carbon (38.9 g).
Calculate the mass ratio of chlorine to carbon for the second sample: divide the mass of chlorine (134 g) by the mass of carbon (14.8 g).
Compare the two mass ratios obtained from the samples. If they are the same or very close, the results are consistent with the law of definite proportions.
Conclude whether the results are consistent with the law of definite proportions based on the comparison of the mass ratios.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Law of Definite Proportions
The Law of Definite Proportions states that a chemical compound always contains its component elements in fixed ratio by mass, regardless of the amount of the compound or its source. For example, water (H2O) always consists of 2 hydrogen atoms and 1 oxygen atom, which translates to a specific mass ratio. This law is fundamental in determining whether the results of the decomposition of carbon tetrachloride are consistent across different samples.
Recommended video:
Guided course
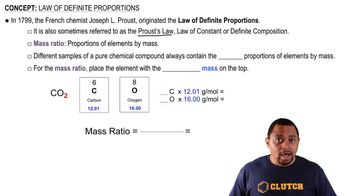
Law of Definite Proportions
Mass Ratio Calculation
To assess consistency with the Law of Definite Proportions, one must calculate the mass ratios of the elements produced from each sample. This involves dividing the mass of carbon by the mass of chlorine for each sample. If the ratios are equivalent, it supports the idea that the samples are consistent with the law; if not, it suggests variability in composition.
Recommended video:
Guided course
Neutron-Proton Ratio
Stoichiometry
Stoichiometry is the branch of chemistry that deals with the quantitative relationships between the reactants and products in a chemical reaction. It allows chemists to predict the amounts of substances consumed and produced in a reaction. Understanding stoichiometry is essential for analyzing the results of the decomposition of carbon tetrachloride, as it helps in determining whether the observed mass ratios align with the expected ratios based on the compound's formula.
Recommended video:
Guided course

Stoichiometry Concept
Related Practice
Textbook Question
1
rank
Textbook Question
An automobile gasoline tank holds 42 kg of gasoline. When the gasoline burns, 168 kg of oxygen is consumed, and carbon dioxide and water are produced. What is the total combined mass of carbon dioxide and water that is produced?
Textbook Question
Upon decomposition, one sample of magnesium fluoride produces 1.65 kg of magnesium and 2.57 kg of fluorine. A second sample produces 2.72 kg of magnesium. How much fluorine (in grams) does the second sample produce?
Textbook Question
The mass ratio of sodium to fluorine in sodium fluoride is 1.21:1. A sample of sodium fluoride produces 45.1 g of sodium upon decomposition. How much fluorine (in grams) forms?
