A Cu/Cu2+ concentration cell has a voltage of 0.22 V at 25 °C. The concentration of Cu2+ in one of the half-cells is 1.5×10–3 M. What is the concentration of Cu2+ in the other half-cell? (Assume the concentration in the unknown cell is the lower of the two concentrations.)
Ch.20 - Electrochemistry

Chapter 20, Problem 89
Refer to the tabulated values of ∆Gf° in Appendix IIB to calculate E°cell for a fuel cell that employs the reaction between methane gas (CH4) and oxygen to form carbon dioxide and gaseous water.
 Verified step by step guidance
Verified step by step guidance1
Identify the balanced chemical equation for the reaction: CH_4(g) + 2O_2(g) \rightarrow CO_2(g) + 2H_2O(g).
Use the standard Gibbs free energy of formation (∆G_f°) values from Appendix IIB for each species involved in the reaction: CH_4(g), O_2(g), CO_2(g), and H_2O(g).
Calculate the standard Gibbs free energy change (∆G°) for the reaction using the formula: ∆G° = Σ(∆G_f° of products) - Σ(∆G_f° of reactants).
Use the relationship between Gibbs free energy change and cell potential: ∆G° = -nFE°_cell, where n is the number of moles of electrons transferred and F is the Faraday constant.
Solve for the standard cell potential (E°_cell) using the equation: E°_cell = -∆G° / (nF).
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Gibbs Free Energy (∆Gf°)
Gibbs Free Energy (∆Gf°) is a thermodynamic potential that measures the maximum reversible work obtainable from a thermodynamic system at constant temperature and pressure. It is crucial for determining the spontaneity of a reaction; a negative ∆Gf° indicates that the reaction can occur spontaneously. In the context of fuel cells, the standard Gibbs free energy change can be used to calculate the cell potential.
Recommended video:
Guided course

Gibbs Free Energy of Reactions
Electrochemical Cell Potential (E°cell)
The standard cell potential (E°cell) is the measure of the voltage produced by an electrochemical cell under standard conditions. It is calculated using the Nernst equation or derived from the Gibbs free energy change using the relationship E°cell = -∆G°/nF, where n is the number of moles of electrons transferred and F is Faraday's constant. Understanding this relationship is essential for evaluating the efficiency of fuel cells.
Recommended video:
Guided course

Electrochemical Cells
Redox Reactions
Redox reactions involve the transfer of electrons between two species, where one species is oxidized (loses electrons) and the other is reduced (gains electrons). In the context of the fuel cell reaction between methane and oxygen, identifying the oxidation states of carbon and oxygen is key to determining the half-reactions and calculating the overall cell potential. Mastery of redox concepts is fundamental for analyzing electrochemical systems.
Recommended video:
Guided course
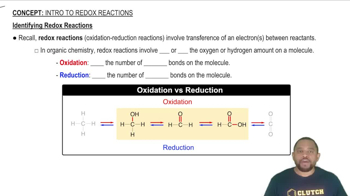
Identifying Redox Reactions
Related Practice
Textbook Question
1
views
Textbook Question
Determine the optimum mass ratio of Zn to MnO2 in an alkaline battery.
Textbook Question
What mass of lead sulfate is formed in a lead–acid storage battery when 1.00 g of Pb undergoes oxidation?
Textbook Question
Refer to the tabulated values of ∆G°f in Appendix IIB to calculate E°cell for the fuel-cell breathalyzer, which employs the following reaction. ((∆G° for HC2H3O2(g) = -374.2 kJ/mol.)
CH3CH2OH(g) + O2(g) → HC2H3O2(g) + H2O(g)
Textbook Question
Determine whether or not each metal, if coated onto iron, would prevent the corrosion of iron. a. Zn
Textbook Question
Determine whether or not each metal, if coated onto iron, would prevent the corrosion of iron. b. Sn
