Textbook Question
Which of these processes is spontaneous? a. the combustion of natural gas b. the extraction of iron metal from iron ore c. a hot drink cooling to room temperature d. drawing heat energy from the ocean's surface to power a ship

 Verified step by step guidance
Verified step by step guidance
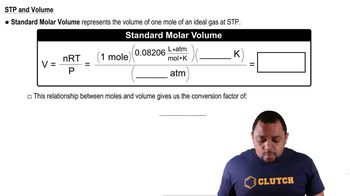

Which of these processes is spontaneous? a. the combustion of natural gas b. the extraction of iron metal from iron ore c. a hot drink cooling to room temperature d. drawing heat energy from the ocean's surface to power a ship
Which of these processes are nonspontaneous? Are the nonspontaneous processes impossible? a. a bike going up a hill b. a meteor falling to Earth c. obtaining hydrogen gas from liquid water d. a ball rolling down a hill