Consider this reaction occurring at 298 K: N2O(g) + NO2(g) ⇌ 3 NO(g) a. Show that the reaction is not spontaneous under standard conditions by calculating ΔG°rxn.

Consider this reaction occurring at 298 K: N2O(g) + NO2(g) ⇌ 3 NO(g) c. Can the reaction be made more spontaneous by an increase or decrease in temperature? If so, what temperature is required to make the reaction spontaneous under standard conditions?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Gibbs Free Energy

Entropy and Enthalpy

Temperature's Effect on Spontaneity
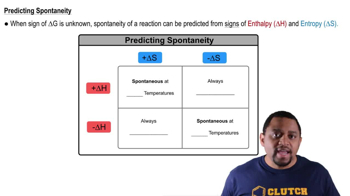
Consider this reaction occurring at 298 K: N2O(g) + NO2(g) ⇌ 3 NO(g) b. If a reaction mixture contains only N2O and NO2 at partial pressures of 1.0 atm each, the reaction will be spontaneous until some NO forms in the mixture. What maximum partial pressure of NO builds up before the reaction ceases to be spontaneous?
Consider this reaction occurring at 298 K: BaCO3(s) ⇌ BaO(s) + CO2(g) a. Show that the reaction is not spontaneous under standard conditions by calculating ΔG°rxn.
Consider this reaction occurring at 298 K: BaCO3(s) ⇌ BaO(s) + CO2( g) b. If BaCO3 is placed in an evacuated flask, what is the partial pressure of CO2 when the reaction reaches equilibrium?
Consider this reaction occurring at 298 K: BaCO3(s) ⇌ BaO(s) + CO2(g) c. Can the reaction be made more spontaneous by an increase or decrease in temperature? If so, at what temperature is the partial pressure of carbon dioxide 1.0 atm?
