Consider the reaction: 2 NO(g) + O2(g) → 2 NO2(g) Estimate ΔG° for this reaction at each temperature and predict whether or not the reaction is spontaneous. (Assume that ΔH° and ΔS° do not change too much within the given temperature range.) b. 715 K
Ch.19 - Free Energy & Thermodynamics

Chapter 19, Problem 73
Consider the sublimation of iodine at 25.0 °C: I2(s) → I2(g). a. Find ΔG°rxn at 25.0 °C.
 Verified step by step guidance
Verified step by step guidance1
Identify the relevant thermodynamic equation: \( \Delta G^\circ_{\text{rxn}} = \Delta H^\circ - T\Delta S^\circ \).
Look up the standard enthalpy change (\( \Delta H^\circ \)) and the standard entropy change (\( \Delta S^\circ \)) for the sublimation of iodine from a reliable source, such as a chemistry textbook or database.
Convert the temperature from Celsius to Kelvin by adding 273.15 to the Celsius temperature: \( T = 25.0 + 273.15 \).
Substitute the values of \( \Delta H^\circ \), \( T \) (in Kelvin), and \( \Delta S^\circ \) into the equation \( \Delta G^\circ_{\text{rxn}} = \Delta H^\circ - T\Delta S^\circ \).
Perform the calculation to find \( \Delta G^\circ_{\text{rxn}} \), ensuring that the units are consistent (e.g., convert \( \Delta S^\circ \) from J/mol·K to kJ/mol·K if necessary).
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Gibbs Free Energy (ΔG)
Gibbs Free Energy (ΔG) is a thermodynamic potential that measures the maximum reversible work obtainable from a thermodynamic system at constant temperature and pressure. It is a crucial concept in predicting the spontaneity of a reaction; a negative ΔG indicates a spontaneous process, while a positive ΔG suggests non-spontaneity. The standard Gibbs free energy change (ΔG°) is calculated under standard conditions, providing a reference point for reactions.
Recommended video:
Guided course
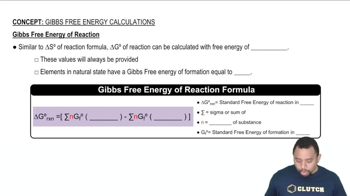
Gibbs Free Energy of Reactions
Sublimation
Sublimation is the phase transition in which a substance changes directly from a solid to a gas without passing through the liquid phase. This process occurs when the molecules in the solid gain enough energy to overcome intermolecular forces and enter the gaseous state. Understanding sublimation is essential for calculating the Gibbs free energy change for the reaction involving iodine, as it directly relates to the enthalpy and entropy changes during the phase transition.
Recommended video:
Guided course
Sublimation Phase Change Example
Standard State Conditions
Standard state conditions refer to a set of specific conditions (usually 1 bar of pressure and a specified temperature, often 25 °C) under which thermodynamic measurements are made. These conditions allow for the comparison of thermodynamic data across different substances. When calculating ΔG°rxn, it is important to use standard state values for enthalpy (ΔH°) and entropy (ΔS°) to ensure accurate results for the reaction at the specified temperature.
Recommended video:
Guided course

Standard Reduction Potentials
Related Practice
Textbook Question
Textbook Question
Determine ΔG° for the reaction: Fe2O3(s) + 3 CO(g) → 2 Fe(s) + 3 CO2(g) Use the following reactions with known ΔG°rxn values:
2 Fe(s) + 3/2 O2(g) → Fe2O3(s) ΔG°rxn = -742.2 kJ
CO(g) + 12 O2( g) → CO2(g) ΔG°rxn = -257.2 kJ
Textbook Question
Consider the sublimation of iodine at 25.0 °C : I2(s) → I2(g) b. Find ΔG°rxn at 25.0 °C under the following nonstandard conditions: i. PI2 = 1.00 mmHg ii. PI2 = 0.100 mmHg
1
views
Textbook Question
Consider the sublimation of iodine at 25.0 °C : I2(s) → I2(g) c. Explain why iodine spontaneously sublimes in open air at 25.0 °C
Textbook Question
Consider the evaporation of methanol at 25.0 °C : CH3OH(l) → CH3OH(g) a. Find ΔG°r at 25.0 °C.
1
views
