For each reaction, calculate ΔH°rxn, ΔS°rxn, and ΔG°rxn at 25 °C and state whether or not the reaction is spontaneous. If the reaction is not spontaneous, would a change in temperature make it spontaneous? If so, should the temperature be raised or lowered from 25 °C? d. 2 KClO3(s) → 2 KCl(s) + 3 O2(g)
Ch.19 - Free Energy & Thermodynamics

Chapter 19, Problem 67
Is the question formulated correctly? If not, please correct it. Here is the question: 'Consider the reaction: 2 NO( g) + O2( g) → 2 NO2( g). Estimate ΔG° for this reaction at each temperature and predict whether or not the reaction is spontaneous, assuming that ΔH° and ΔS° do not change significantly within the given temperature range. a. 298 K b. 855 K.'
 Verified step by step guidance
Verified step by step guidance1
Identify the given reaction: \(2 \text{NO}(g) + \text{O}_2(g) \rightarrow 2 \text{NO}_2(g)\).
Understand that you need to estimate \(\Delta G^\circ\) for the reaction at two different temperatures: 298 K and 855 K.
Recall the Gibbs free energy equation: \(\Delta G^\circ = \Delta H^\circ - T\Delta S^\circ\), where \(T\) is the temperature in Kelvin.
Assume that \(\Delta H^\circ\) and \(\Delta S^\circ\) are constant over the temperature range. You will need these values to calculate \(\Delta G^\circ\) at each temperature.
Calculate \(\Delta G^\circ\) for each temperature using the equation and determine the spontaneity of the reaction: if \(\Delta G^\circ < 0\), the reaction is spontaneous; if \(\Delta G^\circ > 0\), it is non-spontaneous.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Gibbs Free Energy (ΔG)
Gibbs Free Energy (ΔG) is a thermodynamic potential that measures the maximum reversible work obtainable from a thermodynamic system at constant temperature and pressure. It is used to predict the spontaneity of a reaction: if ΔG is negative, the reaction is spontaneous; if positive, it is non-spontaneous. The relationship between ΔG, enthalpy (ΔH), and entropy (ΔS) is given by the equation ΔG = ΔH - TΔS, where T is the temperature in Kelvin.
Recommended video:
Guided course

Gibbs Free Energy of Reactions
Enthalpy (ΔH) and Entropy (ΔS)
Enthalpy (ΔH) is a measure of the total heat content of a system, reflecting the energy required to break and form bonds during a reaction. Entropy (ΔS) quantifies the degree of disorder or randomness in a system. Both ΔH and ΔS are crucial for calculating ΔG and determining the spontaneity of a reaction, as they influence how energy is distributed in the system at different temperatures.
Recommended video:
Guided course

Entropy in Thermodynamics
Temperature's Effect on Spontaneity
Temperature plays a significant role in determining the spontaneity of a reaction through its influence on the Gibbs Free Energy equation. As temperature increases, the TΔS term becomes more significant, which can change the sign of ΔG. Therefore, analyzing the reaction at different temperatures, such as 298 K and 855 K, allows for predictions about whether the reaction will be spontaneous under those conditions.
Recommended video:
Guided course
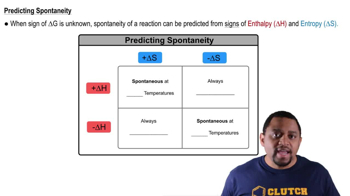
Spontaneity and Temperature
Related Practice
Textbook Question
Textbook Question
Use standard free energies of formation to calculate ΔG° at 25 °C for each reaction in Problem 61. How do the values of ΔG° calculated this way compare to those calculated from ΔH° and ΔS°? Which of the two methods could be used to determine how ΔG° changes with temperature?
1
views
Textbook Question
Consider the reaction: 2 NO(g) + O2(g) → 2 NO2(g) Estimate ΔG° for this reaction at each temperature and predict whether or not the reaction is spontaneous. (Assume that ΔH° and ΔS° do not change too much within the given temperature range.) b. 715 K
Textbook Question
Determine ΔG° for the reaction: Fe2O3(s) + 3 CO(g) → 2 Fe(s) + 3 CO2(g) Use the following reactions with known ΔG°rxn values:
2 Fe(s) + 3/2 O2(g) → Fe2O3(s) ΔG°rxn = -742.2 kJ
CO(g) + 12 O2( g) → CO2(g) ΔG°rxn = -257.2 kJ
