Use the appropriate values of Ksp and Kf to find the equilibrium constant for the reaction. FeS(s) + 6 CN-(aq) ⇌ Fe(CN)64-(aq) + S2-(aq)
Ch.18 - Aqueous Ionic Equilibrium

Chapter 18, Problem 122
A buffer is created by combining 3.55 g of NH3 with 4.78 g of HCl and diluting to a total volume of 750.0 mL. Determine the pH of the buffer.
 Verified step by step guidance
Verified step by step guidance1
Calculate the moles of NH3 using its molar mass: \( \text{moles of NH}_3 = \frac{3.55 \text{ g}}{17.03 \text{ g/mol}} \).
Calculate the moles of HCl using its molar mass: \( \text{moles of HCl} = \frac{4.78 \text{ g}}{36.46 \text{ g/mol}} \).
Determine the limiting reactant by comparing the moles of NH3 and HCl, and calculate the moles of NH4+ formed.
Use the Henderson-Hasselbalch equation: \( \text{pH} = \text{pK}_a + \log \left( \frac{[\text{base}]}{[\text{acid}]} \right) \), where \( \text{pK}_a \) is for NH4+ and the concentrations are based on the remaining NH3 and formed NH4+.
Calculate the concentrations of NH3 and NH4+ by dividing their moles by the total volume of the solution (0.750 L) and substitute these values into the Henderson-Hasselbalch equation to find the pH.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
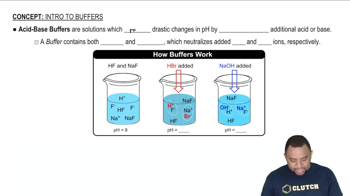
Buffer Solutions
A buffer solution is a system that resists changes in pH upon the addition of small amounts of acid or base. It typically consists of a weak acid and its conjugate base or a weak base and its conjugate acid. In this case, the combination of ammonia (NH3) and hydrochloric acid (HCl) creates a buffer that can maintain a relatively stable pH.
Recommended video:
Guided course
Buffer Solutions
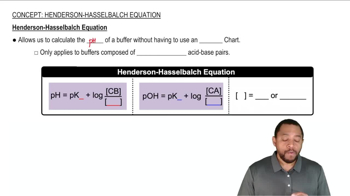
Henderson-Hasselbalch Equation
The Henderson-Hasselbalch equation is a mathematical formula used to calculate the pH of a buffer solution. It is expressed as pH = pKa + log([A-]/[HA]), where pKa is the negative logarithm of the acid dissociation constant, [A-] is the concentration of the base, and [HA] is the concentration of the acid. This equation is essential for determining the pH of the buffer created in the question.
Recommended video:
Guided course
Henderson-Hasselbalch Equation
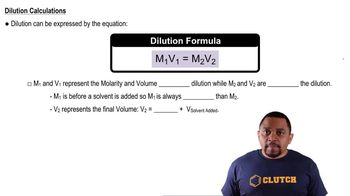
Molarity and Dilution
Molarity is a measure of concentration defined as the number of moles of solute per liter of solution. In this scenario, the total volume of the buffer solution is 750.0 mL, which requires converting grams of NH3 and HCl to moles and then calculating their concentrations. Understanding dilution and how to convert between grams, moles, and volume is crucial for accurately determining the pH of the buffer.
Recommended video:
Guided course
Dilution Equation
Related Practice
Textbook Question
Textbook Question
A 1.0-L buffer solution initially contains 0.25 mol of NH3 and 0.25 mol of NH4Cl. In order to adjust the buffer pH to 8.75, should you add NaOH or HCl to the buffer mixture? What mass of the correct reagent should you add?
Textbook Question
In analytical chemistry, bases used for titrations must often be standardized; that is, their concentration must be precisely determined. Standardization of sodium hydroxide solutions can be accomplished by titrating potassium hydrogen phthalate (KHC8H4O4), also known as KHP, with the NaOH solution to be standardized. b. The titration of 0.5527 g of KHP required 25.87 mL of an NaOH solution to reach the equivalence point. What is the concentration of the NaOH solution?
