Textbook Question
Calculate Kc for each reaction. b. N2(g) + O2(g) ⇌ 2 NO(g) Kp = 4.10×10^–31 (at 298 K)

 Verified step by step guidance
Verified step by step guidance
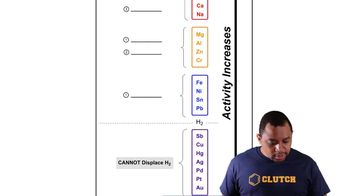
Calculate Kc for each reaction. b. N2(g) + O2(g) ⇌ 2 NO(g) Kp = 4.10×10^–31 (at 298 K)
Calculate Kp for each reaction. a. I2(g) + Cl2(g) ⇌ 2 ICl(g) Kc = 81.9 (at 298 K)
Calculate Kp for each reaction. b. CH4(g) + H2O(g) ⇌ CO(g) + 3 H2(g) Kc = 1.3×10^22 (at 298 K)
Consider the reaction: N2(g) + 3 H2(g) ⇌ 2 NH3(g) Complete the table. Assume that all concentrations are equilibrium concentrations in M.
T (K) [N2] [H2] [NH3] Kc
500 0.115 0.105 0.439 _
575 0.110 _ 0.128 9.6
775 0.120 0.140 _ 0.0584
Consider the following reaction: H2(g) + I2(g) ⇌ 2 HI(g) Complete the table. Assume that all concentrations are equilibrium concentrations in M.
T (°C) [H2] [I2] [HI] Kc
25 0.0355 0.0388 0.922 _
340 _ 0.0455 0.387 9.6
445 0.0485 0.0468 _ 50.2