A reaction in which A, B, and C react to form products is first order in A, second order in B, and zero order in C. b. What is the overall order of the reaction?

A reaction in which A, B, and C react to form products is zero order in A, one-half order in B, and second order in C. c. By what factor does the reaction rate change if [A] is doubled (and the other reactant concentrations are held constant)? d. By what factor does the reaction rate change if [B] is doubled? e. By what factor does the reaction rate change if [C] is doubled? f. By what factor does the reaction rate change if [C] is doubled (and the other reactant concentrations are held constant)?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Order of Reaction

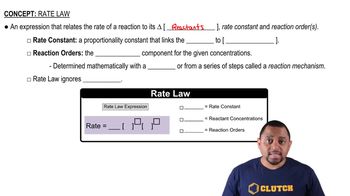
Rate Law
Effect of Concentration Change on Reaction Rate

A reaction in which A, B, and C react to form products is first order in A, second order in B, and zero order in C c. By what factor does the reaction rate change if [A] is doubled (and the other reactant concentrations are held constant)? d. By what factor does the reaction rate change if [B] is doubled (and the other reactant concentrations are held constant)? e. By what factor does the reaction rate change if [C] is doubled? f. By what factor does the reaction rate change if the concentrations of all three reactants are doubled?
A reaction in which A, B, and C react to form products is zero order in A, one-half order in B, and second order in C. a. Write a rate law for the reaction.
Consider the tabulated data showing the initial rate of a reaction (A → products) at several different concentrations of A. What is the order of the reaction? Write a rate law for the reaction including the value of the rate constant, k.
[A] (M) Initial Rate (M/s)
0.050 0.100
0.075 0.225
0.090 0.324
The tabulated data were collected for this reaction: CH3Cl(g) + 3 Cl2(g) → CCl4( g) + 3 HCl(g)
Write an expression for the reaction rate law and calculate the value of the rate constant, k. What is the overall order of the reaction?
