Textbook Question
Pick an appropriate solvent from Table 13.3 to dissolve each substance. State the kind of intermolecular forces that would occur between the solute and solvent in each case. b. sodium chloride (ionic)

 Verified step by step guidance
Verified step by step guidance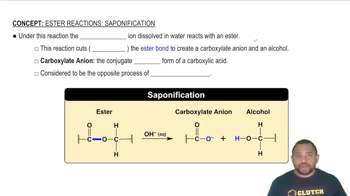

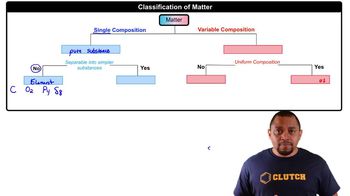
Pick an appropriate solvent from Table 13.3 to dissolve each substance. State the kind of intermolecular forces that would occur between the solute and solvent in each case. b. sodium chloride (ionic)
Which molecule would you expect to be more soluble in water: CH3CH2CH2OH or HOCH2CH2CH2OH?