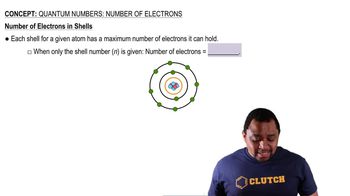
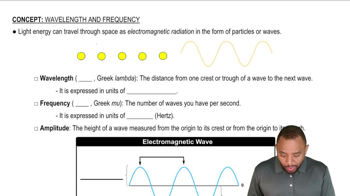
Textbook Question
X-ray diffractometers often use metals that have had their core electrons excited as a source of X-rays. Consider the 2p → 1s transition for copper, which is called the K⍺ transition. Calculate the wavelength of X-rays (in Å) given off by the K⍺ transition if the energy given off by a mole of copper atoms is 7.77⨉105 kJ.(1Å = 10-10 m)
1
views