The vapor pressure of CCl3F at 300 K is 856 torr. If 3.5 g of CCl3F is enclosed in a 1.0-L container, will any liquid be present? If so, what mass of liquid?
Ch.12 - Liquids, Solids & Intermolecular Forces

Chapter 12, Problem 99
Air conditioners not only cool air, but dry it as well. A room in a home measures 6.0 m × 10.0 m × 2.2 m. If the outdoor temperature is 30 °C and the partial pressure of water in the air is 85% of the vapor pressure of water at this temperature, what mass of water must be removed from the air each time the volume of air in the room is cycled through the air conditioner? (Assume that all of the water must be removed from the air.) The vapor pressure for water at 30 °C is 31.8 torr.
 Verified step by step guidance
Verified step by step guidance1
Calculate the volume of the room using the formula: \( \text{Volume} = \text{length} \times \text{width} \times \text{height} \).
Convert the volume from cubic meters to liters, knowing that 1 cubic meter equals 1000 liters.
Determine the partial pressure of water in the air using the given percentage: \( P_{\text{water}} = 0.85 \times 31.8 \text{ torr} \).
Use the ideal gas law to find the number of moles of water vapor in the room: \( PV = nRT \), where \( P \) is the partial pressure of water, \( V \) is the volume in liters, \( R \) is the ideal gas constant (0.0821 L·atm/mol·K), and \( T \) is the temperature in Kelvin.
Convert the moles of water vapor to mass using the molar mass of water (18.02 g/mol).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
8mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Vapor Pressure
Vapor pressure is the pressure exerted by a vapor in equilibrium with its liquid or solid form at a given temperature. It indicates the tendency of particles to escape from the liquid phase into the gas phase. For water at 30 °C, the vapor pressure is 31.8 torr, meaning that at this temperature, water molecules have a specific tendency to evaporate into the air.
Recommended video:
Guided course
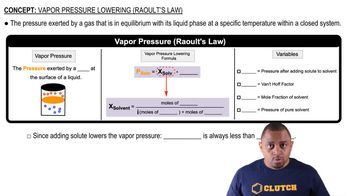
Raoult's Law and Vapor Pressure
Relative Humidity
Relative humidity is the ratio of the current amount of water vapor in the air to the maximum amount of water vapor the air can hold at that temperature, expressed as a percentage. In this scenario, the air has a relative humidity of 85%, indicating that it contains 85% of the maximum water vapor capacity at 30 °C. This concept is crucial for determining how much water can be removed from the air.
Recommended video:
Guided course
Subatomic Particles
Ideal Gas Law
The Ideal Gas Law relates the pressure, volume, temperature, and number of moles of a gas through the equation PV = nRT. This law can be applied to calculate the amount of water vapor in the air by converting the vapor pressure into moles and then determining the mass of water that corresponds to those moles. Understanding this relationship is essential for solving the problem of how much water must be removed from the air.
Recommended video:
Guided course

Ideal Gas Law Formula
Related Practice
Textbook Question
Textbook Question
A sample of steam with a mass of 0.552 g and at a temperature of 100 °C condenses into an insulated container holding 4.25 g of water at 5.0 °C. Assuming that no heat is lost to the surroundings, what is the final temperature of the mixture?
Textbook Question
A sealed flask contains 0.55 g of water at 28 °C. The vapor pressure of water at this temperature is 28.35 mmHg. What is the minimum volume of the flask in order that no liquid water be present in the flask?
Textbook Question
Based on the phase diagram of CO2 shown in Figure 11.39(b), describe the state changes that occur when the temperature of CO2 is increased from 190 K to 350 K at a constant pressure of (a) 1 atm
