Which species has the smaller bond angle, H3O+ or H2O? Explain.

 Tro 6th Edition
Tro 6th Edition Ch.11 - Chemical Bonding II: Molecular Shapes, VSEPR & MO Theory
Ch.11 - Chemical Bonding II: Molecular Shapes, VSEPR & MO Theory Problem 39d
Problem 39dDetermine the molecular geometry and sketch each molecule or ion using the bond conventions shown in “Representing Molecular Geometries on Paper” in Section 11.4. d. BrF5
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
VSEPR Theory

Molecular Geometry

Bonding Conventions
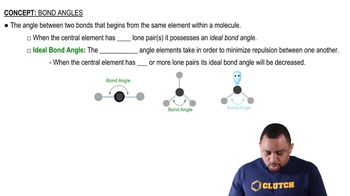
Which species has the smaller bond angle, ClO4- or ClO3- ? Explain.
Determine the molecular geometry and sketch each molecule or ion using the bond conventions shown in “Representing Molecular Geometries on Paper” in Section 11.4. c. PCl5
Determine the molecular geometry and sketch each molecule or ion, using the bond conventions shown in “Representing Molecular Geometries on Paper” in Section 11.4. b. SCl4
Determine the molecular geometry and sketch each molecule or ion, using the bond conventions shown in “Representing Molecular Geometries on Paper” in Section 11.4. c. ClF3
Determine the molecular geometry and sketch each molecule or ion, using the bond conventions shown in “Representing Molecular Geometries on Paper” in Section 11.4. d. IF2–