Use the prefix multipliers to express each measurement without exponents. b. 11 * 10 - 12 s
Ch.1 - Matter, Measurement & Problem Solving

Chapter 1, Problem 56
The warmest temperature ever measured in the United States is 134 °F, recorded on July 10, 1913, in Death Valley, California. Convert that temperature to °C and K.
 Verified step by step guidance
Verified step by step guidance1
First, convert the temperature from Fahrenheit (°F) to Celsius (°C) using the formula: \( C = \frac{5}{9} \times (F - 32) \), where \( F \) is the temperature in Fahrenheit.
Substitute 134 °F into the formula to find the temperature in Celsius.
Next, convert the temperature from Celsius (°C) to Kelvin (K) using the formula: \( K = C + 273.15 \), where \( C \) is the temperature in Celsius.
Substitute the result from step 2 into the formula to find the temperature in Kelvin.
Ensure to check the units in your final answers to confirm they are in °C and K respectively.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Temperature Scales
Temperature can be measured in different scales, primarily Celsius (°C), Fahrenheit (°F), and Kelvin (K). Each scale has its own zero point and degree increments. Understanding how these scales relate to one another is essential for converting temperatures accurately.
Recommended video:
Guided course

The pH Scale
Conversion Formulas
To convert temperatures between Fahrenheit and Celsius, the formula is °C = (°F - 32) × 5/9. For converting Celsius to Kelvin, the formula is K = °C + 273.15. Mastery of these formulas is crucial for performing temperature conversions correctly.
Recommended video:
Guided course

Conversion Factors
Absolute Zero
Kelvin is an absolute temperature scale where 0 K represents absolute zero, the theoretical point where molecular motion ceases. This scale is used in scientific contexts because it provides a direct measure of thermal energy. Understanding this concept helps in grasping the significance of temperature in physical sciences.
Recommended video:
Guided course
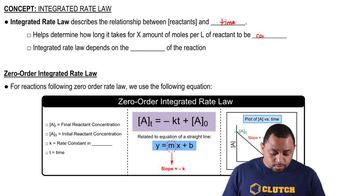
Zero-Order Reactions
Related Practice
Textbook Question
Textbook Question
Classify each change as physical or chemical. a. Natural gas burns in a stove. b. The liquid propane in a gas grill evaporates because the valve was left open. c. The liquid propane in a gas grill burns in a flame. d. A bicycle frame rusts on repeated exposure to air and water.
21
views
1
rank
Textbook Question
Based on the molecular diagram, classify each change as physical or chemical.
26
views
1
rank
Textbook Question
Convert each temperature. a. 32 °F to °C (temperature at which water freezes) b. 77 K to °F (temperature of liquid nitrogen) c. -109 °F to °C (temperature of dry ice) d. 98.6 °F to K (body temperature)
6
views
Textbook Question
Use prefix multipliers to express each measurement without exponents. a. 3.4x10-16m
Textbook Question
Use the prefix multipliers to express each measurement without exponents. c. 7.4 * 103 g
