Use Lewis symbols to determine the formula for the compound that forms between each pair of elements. a. Sr and Se b. Ba and Cl c. Na and S d. Al and O

The lattice energy of CsF is -744 kJ/mol, whereas that of BaO is -3029 kJ/mol. Explain this large difference in lattice energy.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Lattice Energy

Ionic Charge

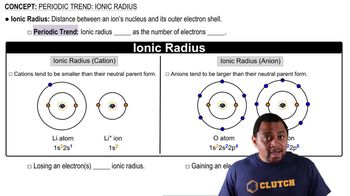
Ionic Radius
Use Lewis symbols to determine the formula for the compound that forms between each pair of elements. a. Ca and N b. Mg and I c. Ca and S d. Cs and F
Rubidium iodide has a lattice energy of -617 kJ/mol, while potassium bromide has a lattice energy of -671 kJ/mol. Why is the lattice energy of potassium bromide more exothermic than the lattice energy of rubidium iodide?
Use the Born–Haber cycle and data from Appendix IIB, Chapter 9 and this chapter to calculate the lattice energy of KCl. (ΔHsub for potassium is 89.0 kJ>mol.)
Use the Born–Haber cycle and data from Appendix IIB and Table 10.3 to calculate the lattice energy of CaO. (ΔHsub for calcium is 178 kJ>mol; IE1 and IE2 for calcium are 590 kJ>mol and 1145 kJ>mol, respectively; EA1 and EA2 for O are -141 kJ>mol and 744 kJ>mol, respectively.)
