If hydrogen were used as a fuel, it could be burned according to this reaction: H2(g) + 1/2 O2(g) → H2O(g) Use average bond energies to calculate ΔHrxn for the combustion of methane (CH4).
Ch.10 - Chemical Bonding I: The Lewis Model

Chapter 10, Problem 106
Calculate ΔHrxn for the combustion of octane (C8H18), a component of gasoline, by using average bond energies and then calculate it using enthalpies of formation from Appendix IIB. What is the percent difference between your results? Which result would you expect to be more accurate?
 Verified step by step guidance
Verified step by step guidance1
Identify the balanced chemical equation for the combustion of octane, which is: C8H18(l) + 12.5 O2(g) → 8 CO2(g) + 9 H2O(l).
Calculate ΔHrxn using average bond energies: First, sum up the bond energies for all the bonds broken in the reactants (C-H, C-C, and O=O bonds) and then sum up the bond energies for all the bonds formed in the products (C=O and O-H bonds). ΔHrxn can be calculated using the formula ΔHrxn = Σ(Bond Energies of Bonds Broken) - Σ(Bond Energies of Bonds Formed).
Calculate ΔHrxn using enthalpies of formation: Use the formula ΔHrxn = Σ(ΔHf° of products) - Σ(ΔHf° of reactants), where ΔHf° values can be found in Appendix IIB for CO2(g), H2O(l), C8H18(l), and O2(g).
Calculate the percent difference between the two ΔHrxn values obtained from bond energies and enthalpies of formation using the formula: Percent Difference = |(Value1 - Value2) / Average of Value1 and Value2| × 100%.
Discuss which result is more accurate: The calculation using enthalpies of formation is generally more accurate because it is derived from experimental data specific to the substances involved, whereas bond energies are average values that might not precisely reflect the specific molecule.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
11mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Bond Energies
Bond energies represent the amount of energy required to break a bond between two atoms in a molecule. In the context of combustion reactions, average bond energies can be used to estimate the enthalpy change (ΔHrxn) by calculating the energy required to break the bonds in the reactants and the energy released when new bonds are formed in the products. This method provides a rough estimate of the reaction's energy change.
Recommended video:
Guided course
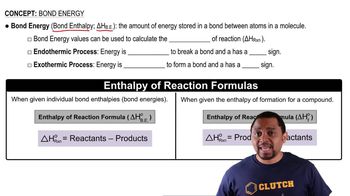
Bond Energy
Enthalpy of Formation
The enthalpy of formation (ΔHf) is the change in enthalpy when one mole of a compound is formed from its elements in their standard states. This value is crucial for calculating the overall enthalpy change of a reaction using Hess's law, which states that the total enthalpy change for a reaction is the sum of the enthalpy changes for individual steps. Using enthalpies of formation typically yields more accurate results than bond energy calculations.
Recommended video:
Guided course

Enthalpy of Formation
Percent Difference
Percent difference is a way to express the difference between two values as a percentage of their average. It is calculated using the formula: |Value1 - Value2| / [(Value1 + Value2) / 2] × 100%. In this context, it helps quantify the discrepancy between the ΔHrxn values obtained from bond energies and enthalpies of formation, providing insight into the accuracy of the methods used.
Recommended video:
Guided course

Mass Percent Example
Related Practice
Textbook Question
Textbook Question
If hydrogen were used as a fuel, it could be burned according to this reaction: H2(g) + 1/2 O2(g) → H2O(g) Which fuel yields more energy per mole?
Textbook Question
If hydrogen were used as a fuel, it could be burned according to this reaction: H2(g) + 1/2 O2(g) → H2O(g) Which fuel yields more energy per gram?
Textbook Question
Draw the Lewis structure for each compound. b. H3PO3 (two OH bonds)
Textbook Question
Draw the Lewis structure for each compound. c. H3AsO4
Textbook Question
The azide ion, N3-, is a symmetrical ion, all of whose contributing resonance structures have formal charges. Draw three important contributing structures for this ion.
