Textbook Question
Consider this set of ionization energies. IE1 = 578 kJ/mol IE2 = 1820 kJ/mol IE3 = 2750 kJ/mol IE4 = 11,600 kJ/mol To which third-period element do these ionization values belong?
2
views

 Verified step by step guidance
Verified step by step guidance
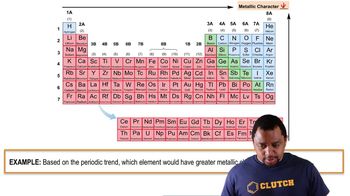


Consider this set of ionization energies. IE1 = 578 kJ/mol IE2 = 1820 kJ/mol IE3 = 2750 kJ/mol IE4 = 11,600 kJ/mol To which third-period element do these ionization values belong?
Choose the element with the more negative (more exothermic) electron affinity from each pair. a. Na or Rb
Choose the more metallic element from each pair. c. Cl or O
Arrange these elements in order of increasing metallic character: Fr, Sb, In, S, Ba, Se.
Arrange these elements in order of decreasing metallic character: Sr, N, Si, P, Ga, Al.