The speed of sound in air is 344 m/s at room temperature. The lowest frequency of a large organ pipe is 30 s–1 and the highest frequency of a piccolo is 1.5×104 s–1. Find the difference in wavelength between these two sounds.
Ch.8 - The Quantum-Mechanical Model of the Atom

Chapter 8, Problem 91
A laser produces 20.0 mW of red light. In 1.00 hr, the laser emits 2.29×1020 photons. What is the wavelength of the laser?
 Verified step by step guidance
Verified step by step guidance1
Identify the given values: Power (P) = 20.0 mW, Time (t) = 1.00 hr, Number of photons (N) = 2.29 \times 10^{20}.
Convert the power from milliwatts to watts: 1 mW = 0.001 W, so 20.0 mW = 0.020 W.
Convert the time from hours to seconds: 1 hr = 3600 s, so 1.00 hr = 3600 s.
Calculate the total energy emitted by the laser using the formula: Energy (E) = Power (P) \times Time (t).
Use the energy of a single photon formula: E_{photon} = \frac{E}{N}, and relate it to wavelength using E_{photon} = \frac{hc}{\lambda}, where h is Planck's constant and c is the speed of light, to solve for the wavelength \lambda.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
5mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Photon Energy
The energy of a photon is given by the equation E = hν, where E is energy, h is Planck's constant (6.626 x 10^-34 J·s), and ν (nu) is the frequency of the light. The frequency can be related to the wavelength (λ) using the equation c = λν, where c is the speed of light (approximately 3.00 x 10^8 m/s). Understanding photon energy is essential for calculating the wavelength from the number of emitted photons.
Recommended video:
Guided course
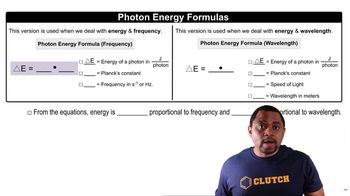
Photon Energy Formulas
Power and Energy Relationship
Power is defined as the rate at which energy is emitted or transferred. In this context, the laser's power output (20.0 mW) indicates how much energy is emitted per unit time. To find the total energy emitted over a specific time period, you can multiply the power by the time (in seconds). This relationship is crucial for determining the total energy associated with the emitted photons.
Recommended video:
Guided course
Power and Root Functions Example
Wavelength Calculation
Wavelength can be calculated using the relationship between energy and frequency. Once the total energy emitted by the laser is determined, the energy per photon can be found by dividing the total energy by the number of photons emitted. This energy can then be used to find the wavelength using the equation λ = hc/E, where E is the energy per photon. This process is key to solving the question regarding the wavelength of the laser light.
Recommended video:
Guided course

Frequency-Wavelength Relationship
Related Practice
Textbook Question
2
views
Textbook Question
The distance from Earth to the sun is 1.5×108 km. Find the number of crests in a light wave of frequency 1.0×1014 s –1 traveling from the sun to Earth.
Textbook Question
A 5.00-mL ampule of a 0.100-M solution of naphthalene in hexane is excited with a flash of light. The naphthalene emits 15.5 J of energy at an average wavelength of 349 nm. What percentage of the naphthalene molecules emitted a photon?
1
views
Textbook Question
A particular laser consumes 150.0 watts of electrical power and produces a stream of 1.33×1019 1064-nm photons per second. What is the percent efficiency of the laser in converting electrical power to light?
1
views
