Zinc metal reacts with hydrochloric acid according to the balanced equation: Zn(s) + 2 HCl(aq) → ZnCl2(aq) + H2(g) When 0.103 g of Zn(s) is combined with enough HCl to make 50.0 mL of solution in a coffee-cup calorimeter, all of the zinc reacts, raising the temperature of the solution from 22.5 °C to 23.7 °C. Find ΔHrxn for this reaction as written. (Use 1.0 g/mL for the density of the solution and 4.18 J/g•°C as the specific heat capacity.)

For each generic reaction, determine the value of ΔH2 in terms of ΔH1.
a. A + B → 2 C ΔH1
2 C→ A + B ΔH2 = ?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Enthalpy Change (ΔH)

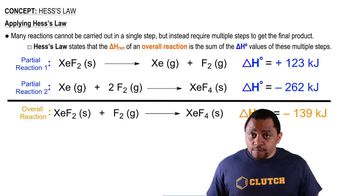
Hess's Law
Reversible Reactions

Determine whether each process is exothermic or endothermic and indicate the sign of ΔH. a. natural gas burning on a stove b. isopropyl alcohol evaporating from skin c. water condensing from steam Indicate the sign of ΔH for the following processes.
Instant cold packs used to ice athletic injuries on the field contain ammonium nitrate and water separated by a thin plastic divider. When the divider is broken, the ammonium nitrate dissolves according to the endothermic reaction: NH4NO3(s) → NH4+(aq) + NO3– (aq) In order to measure the enthalpy change for this reaction, 1.25 g of NH4NO3 is dissolved in enough water to make 25.0 mL of solution. The initial temperature is 25.8 °C and the final temperature (after the solid dissolves) is 21.9 °C. Calculate the change in enthalpy for the reaction in kJ. (Use 1.0 g/mL as the density of the solution and 4.18 J/g•°C as the specific heat capacity.)
For each generic reaction, determine the value of ΔH2 in terms of ΔH1.
b. A + 1/2 B → C ΔH1
2 A + B → 2 C ΔH2 = ?
For each generic reaction, determine the value of ΔH2 in terms of ΔH1.
c. A → B + 2 C ΔH1
1/2 B + C → 1/2 A ΔH2 = ?
Consider the generic reaction:
A + 2 B → C + 3 D ΔH = 155 kJ
Determine the value of ΔH for each related reaction.
a. 3 A + 6 B → 3 C + 9 D
b. C + 3 D → A + 2 B
c. 1/2 C + 3/2 D → 1/2 A + B
