Write balanced complete ionic and net ionic equations for each reaction. CaS(aq) + CuCl2(aq) → CuS(s) + CaCl2(aq)

Write balanced complete ionic and net ionic equations for each reaction. a. K2SO4(aq) + CaI2(aq) → CaSO4(s) + KI(aq)
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
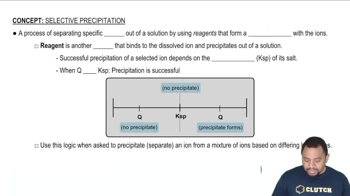
Key Concepts
Complete Ionic Equation

Net Ionic Equation

Precipitation Reaction
Write balanced complete ionic and net ionic equations for each reaction.
c. NaOH(aq) + HC2H3O2(aq) → H2O(l ) + NaC2H3O2(aq)
d. Na3PO4(aq) + NiCl2(aq) → Ni3(PO4)2(s) + NaCl(aq)
Write balanced complete ionic and net ionic equations for each reaction. d. Na3PO4(aq) + NiCl2(aq) → Ni3(PO4)2(s) + NaCl(aq)
Write balanced complete ionic and net ionic equations for each reaction.
a. K2SO4(aq) + CaI2(aq) → CaSO4(s) + KI(aq)
b. NH4Cl(aq) + NaOH(aq) → H2O(l) + NH3(g) + NaCl(aq)
c. AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq)
Write balanced complete ionic and net ionic equations for each reaction. d. HC2H3O2(aq) + K2CO3(aq) → H2O(l ) + CO2(g) + KC2H3O2(aq)
Mercury(I) ions (Hg22+) can be removed from solution by precipitation with Cl- Suppose that a solution contains aqueous Hg2(NO3)2. Write complete ionic and net ionic equations for the reaction of aqueous Hg2(NO3)2 with aqueous sodium chloride to form solid Hg2Cl2 and aqueous sodium nitrate.
